Phosphorus
Phosphorus:

Commonly used ‘phosphate’ plant fertiliser can be applied in its most basic form as crystalline diammonium hydrogen phosphate.
Facts about Phosphorus:
- Phosphorus: white, black, red or violet solid, depending on the type.
- Fun fact about Phosphorus: Phosphorus has been called ‘the devils element’ for many different reasons over the centuries – not only was flaming phosphorus a terrible weapon in the second world war, is highly toxic to the liver if ingested and a key ingredient in nerve gas, but it was also the 13th element to be discovered!
- Chemical symbol: P
- Atomic number: 15
A crystal structure containing Phosphorus:
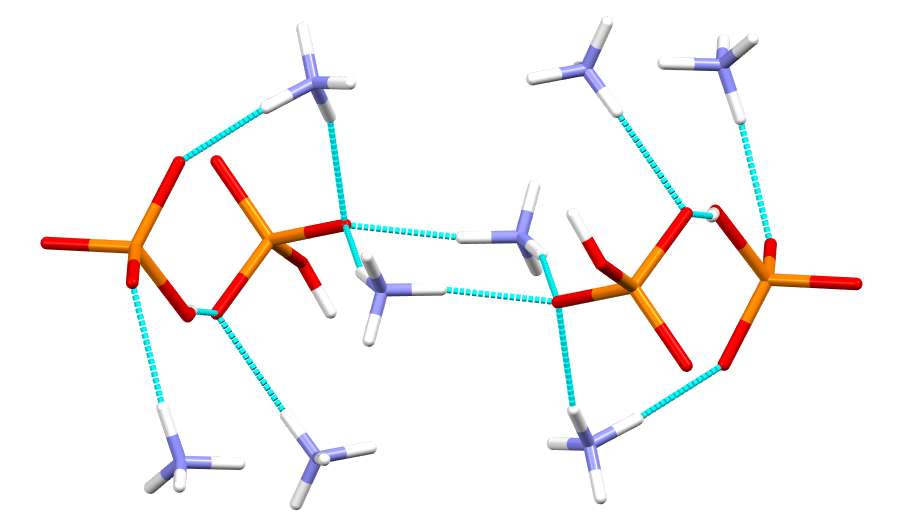
The N-H…O hydrogen bonding network that binds ammonium and phosphate in the crystal structure (note: there is also an O-H…O interaction between two phosphate molecules)
Facts about this structure:
- Formula: H9 N2 O4 P
- Structure name: diammonium hydrogen phosphate
- Fun fact about the structure: The first crystallographic investigation of diammonium hydrogen phosphate was performed in 1957, but only unit cell dimensions and space group were determined. The isomorphous arsenate structure was determined in 1970, but the hydrogen atoms could not be located, so it was only when this structure was determined in 1972 that the hydrogen bonding network that holds together this series of important compounds was unambiguously understood.
- ICSD number: 2799 (Find out more about the ICSD database)
- Associated publication: A.A. Khan, J.P. Roux, W.J. James, Acta Crystallographica,Section B:Struct.Crystallogr.Cryst.Chem., 1972, 28, 2065, DOI: 10.1107/S0567740872005539
More about Phosphorus:
Diammonium Hydrogen Phosphate is an exceptionally versatile material. It is primarily known and used as a fertiliser – it temporarily increases the soil pH, which then lowers over time as nitrification of the ammonium occurs. It is also heavily used in the baking industry as a synergistic ingredient in breadmaking – providing phosphorus and nitrogen which are vital for yeast cell growth. It is for this reason that it is also used in the winemaking industry! It is also used widely as a fire retardant in fighting forest fires, while other documented uses include being a cigarette additive, a sugar purification agent, a flux for soldering and a control for the precipitation of dyes on wool!
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Phosphorus in real crystal structures: