Supersized Aromatic Me
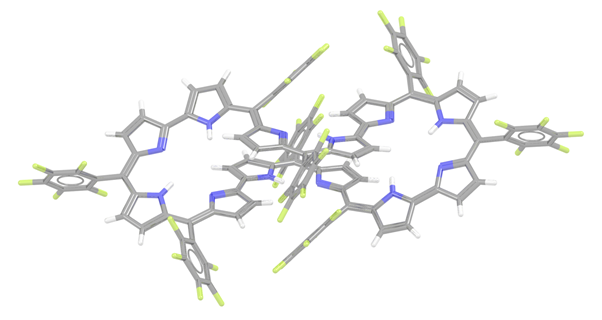
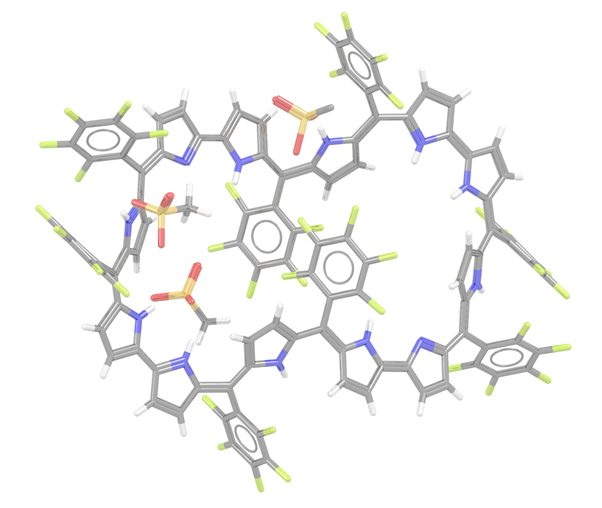
I recently came across a report about the largest known aromatic molecule, to date,1 and it got me thinking about similar entries in the CSD. I know from editing structures into the database that a significant proportion of new entries contain an aromatic system. The structures also seem to be getting bigger and bigger but I had never really connected these two features in any detail. First of all, I was delighted to learn that the supersized structures from the report were already in the CSD. Our new automated systems could deal with them without a problem and one of my colleagues had already cast their expert eye over them. In the report Dongho Kim and co-workers synthesised a [50]dodecaphyrin (KUHHIG, see below), the full name of which is in our curated entry but would have taken up half this blog so I decided not to include it! If you are wondering how to make your own supersized aromatic then the structure was synthesised to contain Hückel aromaticity by oxidising a non-aromatic [52]dodecaphyrin with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ). Dongho Kim and co-workers appear to be record breakers in the world of these structures; the largest known aromatic prior to this was a [46]decaphyrin palladium(II) complex (DONZEN), also synthesised by the same research groups.2
- Soya, T., Kim, W., Kim, D. and Osuka, A. Chemistry – A European Journal, 2015, DOI: 10.1002/chem.201500650. Stable [48]-, [50]-, and [52]Dodecaphyrins(1.1.0.1.1.0.1.1.0.1.1.0): The Largest Hückel Aromatic Molecules.
- Yoneda, T.; Sung, Y. M.; Lim, J. M.; Kim, D. and Osuka, A. Angewandte Chemie International Edition, 2014, 53, 13169, DOI: 10.1002/anie.201408506. PdII Complexes of [44]- and [46]Decaphyrins: The Largest Hückel Aromatic and Antiaromatic, and Möbius Aromatic Macrocycles.
- http://www.pharmacytimes.com/publications/issue/2013/July2013/Top-200-Drugs-of-2012