CSP Blind Tests
CSP or Crystal Structure Prediction, is the science of predicting the crystal structure of a molecule given only its 2D molecular structure. The CCDC CSP Blind Test brings together researchers at the forefront of this field to test their methods against real targets.
Crystal Structure Prediction Blind Tests
Since 1999 the CCDC has held Crystal Structure Prediction (CSP) Blind Tests to bring together scientists in this field and advance methods.
This complex field can potentially reduce risks and generate new ideas in various fields such as drug design. Factors such as molecular conformation, packing patterns, and varying polymorphic forms mean it is not simple to go from a 2D molecular structure to a 3D crystal structure.
The CSP Blind Test brings together scientists in the field from industry and academia to test their methods against a real example in a controlled environment and make connections in the CSP community.
Update October 2024
The results and conclusions of the 7th CSP Blind Test have been published in Acta Crystallographica:
How Does the CSP Blind Test Work?
A selection of small molecules which have been solved experimentally, but remain unpublished, are selected by the organisers. Only their 2D molecular structure and solvate conditions are released to participants.
The participants have 1 year to make their predictions and submit them to the organisers.
At the end of the test period, structures are revealed and each prediction is compared to the experimentally determined results.
The end of test meeting allows all participants and organisers to discuss and reflect on the results and learnings which can be taken forward into future work.
Who Takes Part in the CSP Blind Test?
Participants in the CSP blind test include scientists working on CSP methods from academic, industrial and contract research organisations (CRO’s).
Video: Introduction to Crystal Structure Prediction
In this short video Jason Cole outlines what crystal structure prediction is, its impact and relevance in industrial settings such as pharmaceutical research, and introduces us to the CCDC CSP Blind
Test.
Past CSP Blind Tests
Sixth CSP Blind Test - 2015
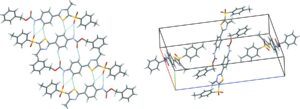
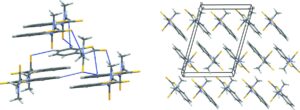
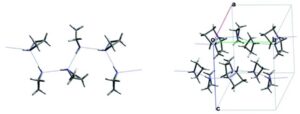
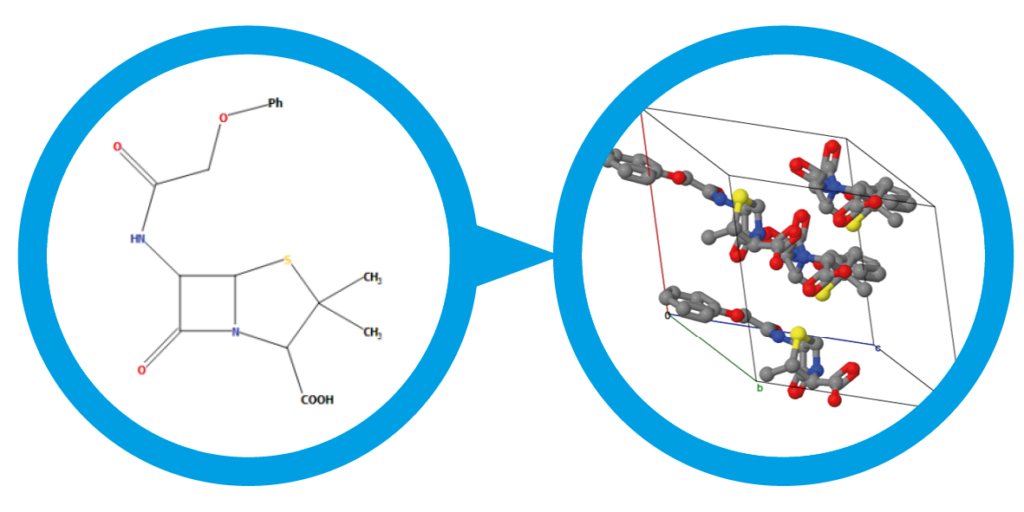
The sixth blind test ended in August 2015 and was the biggest to date, with 25 different submissions and over 90 researchers involved. The five target systems were a small relatively rigid molecule, a former drug candidate, with five known but unpublished polymorphs, a salt hydrate, a co-crystal, and the largest molecule in a blind test to date. All of the target systems were predicted, apart from a single Z’ = 2 polymorph of the drug candidate. Overall, the results are encouraging for the development and maturity of CSP methods, which are now applicable to larger molecules and more complex solid forms such as hydrates and salts.
The full results of the sixth blind test are published in a special issue of Acta Cryst. B.
Target XXIV in the six blind test of organic CSP methods, which is a chloride salt hydrate of (Z)-3-((diaminomethyl)thio)acrylic acid.