Behind the scenes of the CSP Blind Test
How many groups took part in the first challenge of the 7th CSP Blind Test? What kind of experimental work goes on behind the scenes? Read on for answers, and an interesting Q&A with a team from Catalent. Catalent is a development and manufacturing partner that has provided the high-quality experimental data for this test of crystal structure prediction (CSP) methods.
An update on the stoichiometry challenge
We challenged teams to use state-of-the-art CSP methods to predict the stoichiometry of a co-crystal with only the 2D structure of the two components. The deadline for this stoichiometry challenge has now passed. And while we can’t comment on the analysis of this new and exciting challenge just yet (roll-on 2022!), we can say that we received submissions from 13 participating teams! In the meantime, let’s take a closer look at one of the target molecules for the CSP Blind Test – Target Compound XXX – and the CRO that provided the structure through an experimental polymorph screen of the co-crystal system.
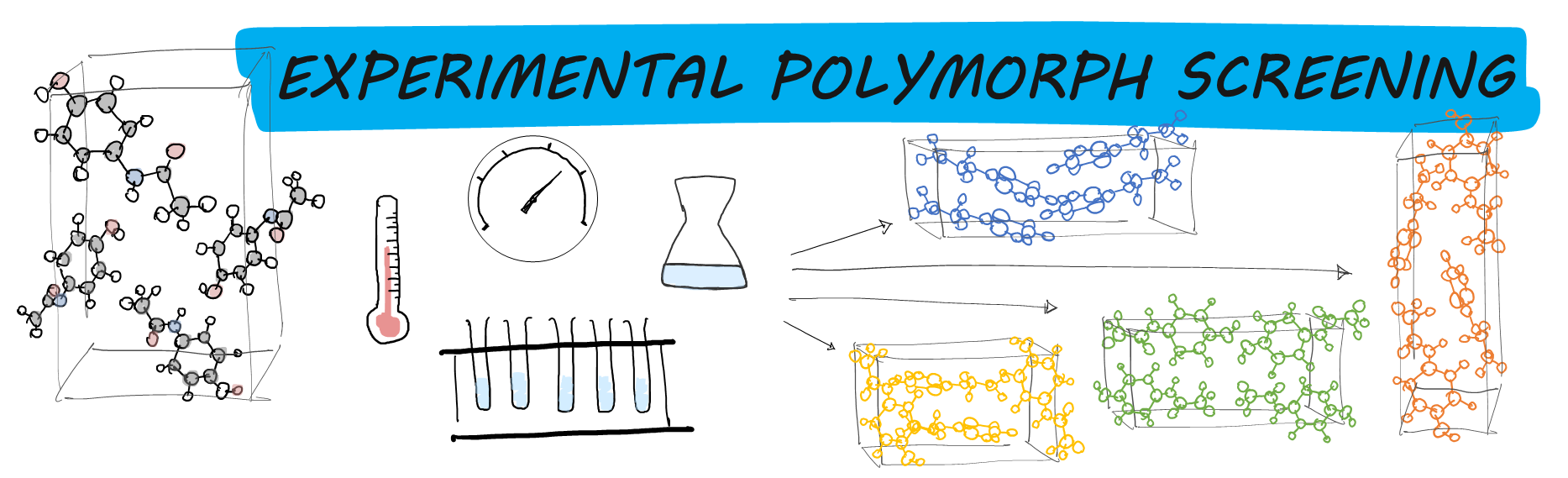
A bit about experimental polymorph screening
Nowadays, it is generally accepted that every molecular crystal system is polymorphic. Often, the greater the effort spent searching for polymorphs, the greater the number of known polymorphs of a system. (I.e., the more you look, the more you find!) For this reason, the objective of the CSP Blind Test is not for CSP methods to find the crystal structure (as it was back in 1999) but to predict different possible polymorphs of a given system.

Before we analyse how successful CSP methods are in doing this, we must ensure we have sufficiently looked. So for each target compound featured in the CSP Blind Test, we have arranged for a complete experimental polymorph screen. (Some systems had a great deal of experimental work already carried out thanks to the providers!) For a bit on the what and why behind experimental polymorph screening, take a look at the graphic below.

A Q&A with the experimental data providers, Catalent
A solid-state group from Catalent is carrying out experimental work for the current CSP Blind Test. Catalent is a development and manufacturing partner that also provides experimental data to industries such as pharmaceutical, biologics and consumer health. Here, the team from Catalent carried out a polymorph screen on target XXX. We caught up with Dr Joanna Bis and Dr Stephen Carino who led the experimental work to hear their thoughts on the significance of a polymorph screen in this case, and how a positive outcome from this Blind Test challenge may aid in experimental work.
What significance does a polymorph screen have for this target compound and the stoichiometry challenge?
The target compound is intended for pharmaceutical application and for such purpose, having a good understanding of both the polymorphic landscape and stoichiometric control will allow for a more robust and reproducible manufacturing process of the cocrystal. A favourable result from the CSP will be an excellent affirmation of the experimental work and vice versa.
How would the prediction of stoichiometry by CSP aid in the experimental screening work you routinely carry out? (What role would it play?)
Having upfront crystal structure and stoichiometry predictions would help in targeting the preferred composition in the search of cocrystals (or salts). Knowledge of the likely interaction motifs, as well as stoichiometry of components, can be taken into consideration during the design stage and thus the screen can be focused on a smaller set of reagents that possess specific moieties and their potential stoichiometry (mono-, di-, etc.). This could reduce the amount of experimental labour, material usage and time spent, ultimately offering an opportunity for cost-saving to the project.
What kind of challenge would you like to see in a future CSP Blind Test and why?
A couple of challenges comes to mind: (1) improving temperature dependence of relative stabilities of polymorphs would help in identifying enantiotropic relationships; and (2) identification of solvates of the API (which we would imagine will involve methodologies analogous to CSP Target XXX). Both of these are critical considerations during crystallization process development and is, therefore, highly desirable that these risks are identified and discharged early on to avoid potential scale-up issues.