CSD Tools in Action: Cocrystal Design and Structural Features Analysis
Here we highlight a paper by Izabela D. Madura and co-workers from the Warsaw University of Technology, where the CCDC tools for crystal structure analysis are used to design and study two cocrystals of caffeine and phenylboronic acid.
You will learn about:
- What steps should be taken to design new cocrystals and to perform a thorough analysis of their structural features;
- Which CCDC tools can be used at each step of a cocrystals study and investigation.
Why?
Cocrystallization occurs when two or more different molecular or ionic compounds combine within a crystal lattice through noncovalent interactions. This method is often used to improve the properties of active compounds, an aspect that is particularly important for drug molecules.
In this study, two cocrystal polymorphs composed of caffeine and 4-chlorophenylboronic acid are investigated using the solid-state informatics tools offered by the CCDC. An analysis of the chosen components is performed, followed by details of the approach used to design the new cocrystals. Finally, their synthesis is presented, and a comparison of the obtained crystal structures is performed.
Each step of this investigation was performed using one or more of the CCDC tools, demonstrating their utility in analysing cocrystal polymorphs and their ability to identify and describe important structural features.
How?
The CSD Search
The first step to design the new cocrystals was a search of the two components, caffeine and phenylboronic acids, in the Cambridge Structural Database (CSD).
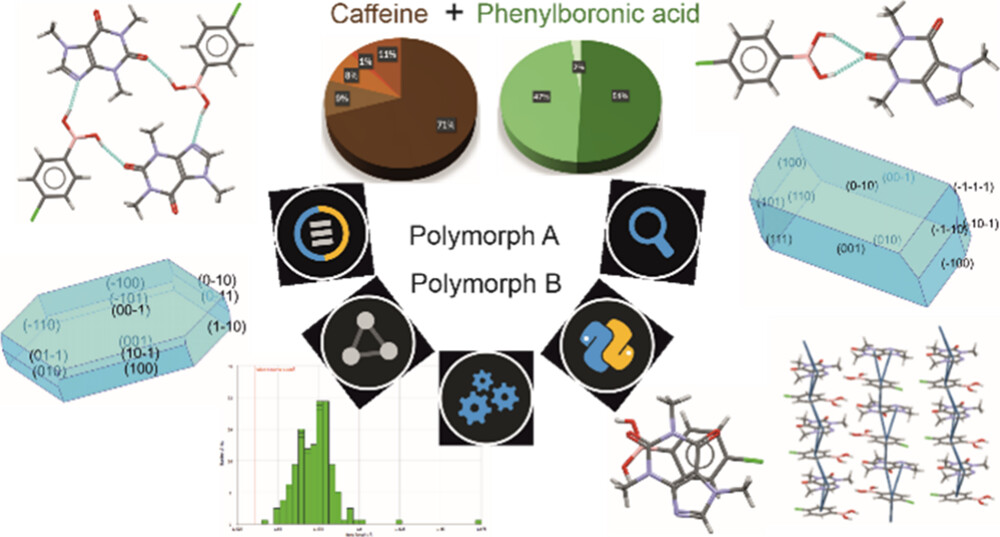
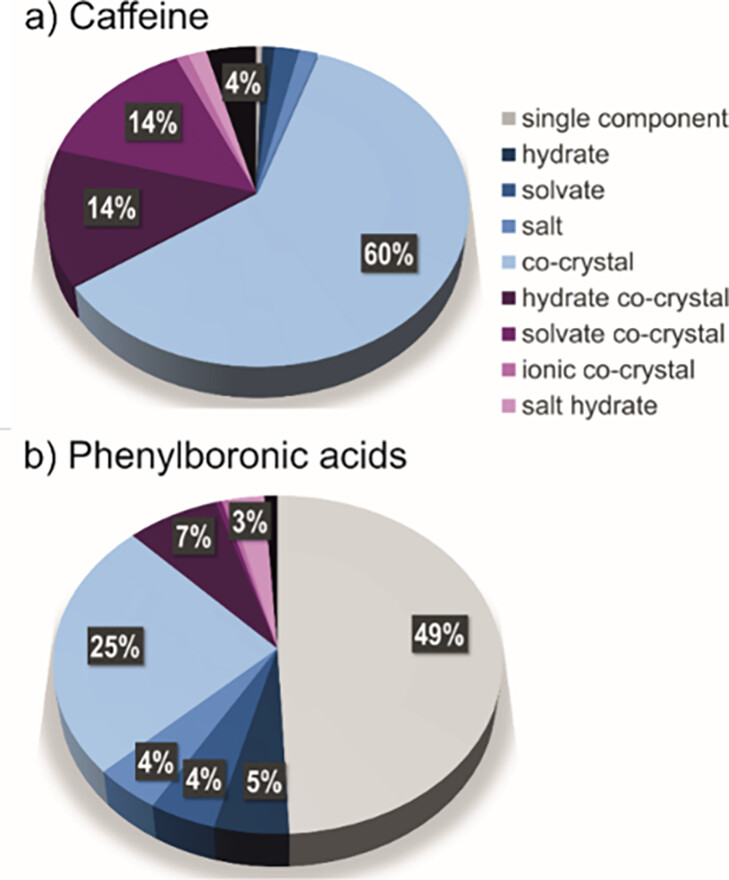
While almost 90% of the crystal forms of caffeine found in the CSD constitute cocrystals (Figure 1, top), most of the structures of phenylboronic acids are instead single-component crystals, and only 35% are cocrystals (Figure 1, bottom).
Aiming to rationalize these differences, the team analysed the frequency of participation of the acceptors present in these molecules involved in hydrogen bonds with OH donors using the Motifs Search available in Mercury. The scientists also investigated the stoichiometry of the searched cocrystals, and the preferred dimensionality of the structures extracted in this query.
As a result of these analyses, it was shown that in caffeine cocrystals most of the hydrogen bonds involve an imidazole nitrogen atom as acceptor (Figure 2, bottom). Caffeine also preferentially forms cocrystals with a 1:1 stoichiometry, and low dimensionality is preferred over the formation of rings and chains.
On the other hand, in phenylboronic acids, the boronic group can donate to two hydrogen bonds, and the three possible conformations of this group makes it hard to predict how the cocrystal system may result (Figure 2, top). On top of that, the boronic group can also accept two hydrogen bonds, resulting in a greater variety of structures available. Despite this, the search of the CSD suggested that phenylboronic acids are more likely to form synthons with molecules having nitrogen atoms as acceptors, and a 1:1 stoichiometry is preferred in this case too.
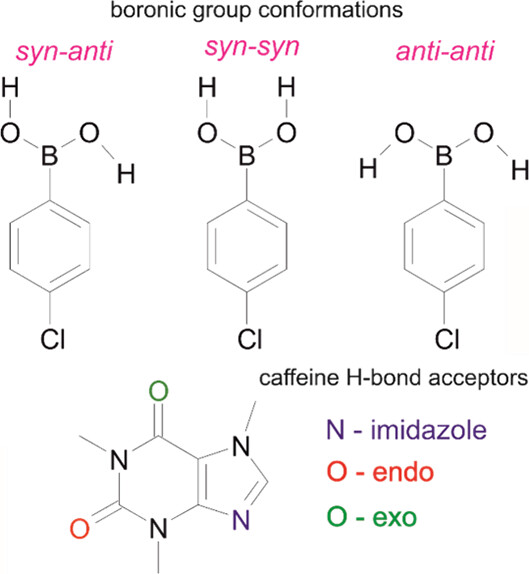
In conclusions, given the complementarity of the functional groups in caffeine and phenylboronic acids, it is likely that the two components will form cocrystals with a 1:1 stoichiometry. However, as a variety of conformations are accessible, the supramolecular packing may be hard to predict.
The Cocrystal Design
Moving the focus of this analysis specifically to the 4-chlorophenylboronic acid molecule, the team performed a thorough analysis of CSD Entry ULUQUP, a cocrystal of 4-chlorophenylboronic acid with theophylline.
Structurally speaking, theophylline is very similar to caffeine, and only differs for the presence of an N‒H bond rather than the N‒CH3 one found in the imidazole ring of caffeine. It was found that in CSD Entry ULUQUP, the theophylline molecule interacts with the boronic group via the imidazole nitrogen and the endo-oxygen atoms, forming a pseudotetrameric motif (Figure 3).
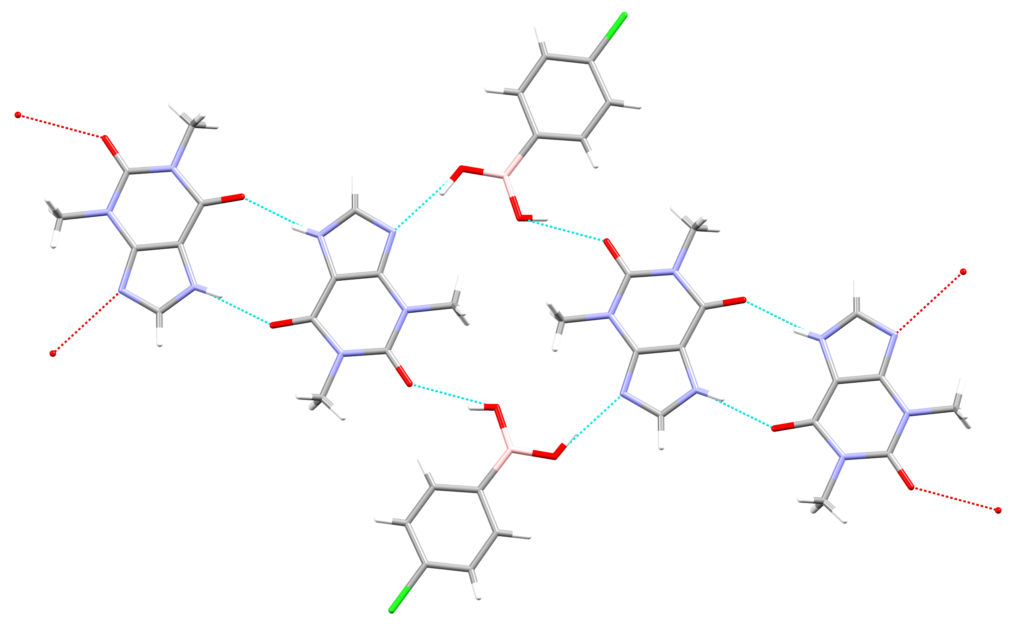
Using the Crystal Packing Similarity search, the team found that this motif is comparable to the one seen in the structures involving 3-hydroxybenzoic acid and theophylline (DOPMUS), and 3-hydroxybenzoic acid and caffeine (MOZCOU).
From the Full Interaction Maps (FIMs) calculation, the team also confirmed that theophylline and caffeine are quite similar in terms of predicted locations of interactions with certain functional groups.
All these observations supported the hypothesis for 4-chlorophenylboronic acid and caffeine to form cocrystals. The pseudotetrameric motif seen above seemed to be the most likely to form, but it isn’t the only one available.
The Cocrystals Synthesis and Study
The synthesis of the cocrystal of 4-chlorophenylboronic acid and caffeine was hence performed and led to the isolation of two polymorphs (polymorph A and B).
Figure 4 shows that the pseudotetrameric motif predicted from the analysis discussed above is present in both the polymorphs. More specifically, polymorph A (Figure 4, top) exhibits two of these centrosymmetric motifs. Polymorph B (Figure 4, bottom) exhibits the expected pseudotetrameric motif, and a bidentate H-bonded motif (Figure 4, bottom, II) that has not been observed before in phenylboronic acid cocrystals.
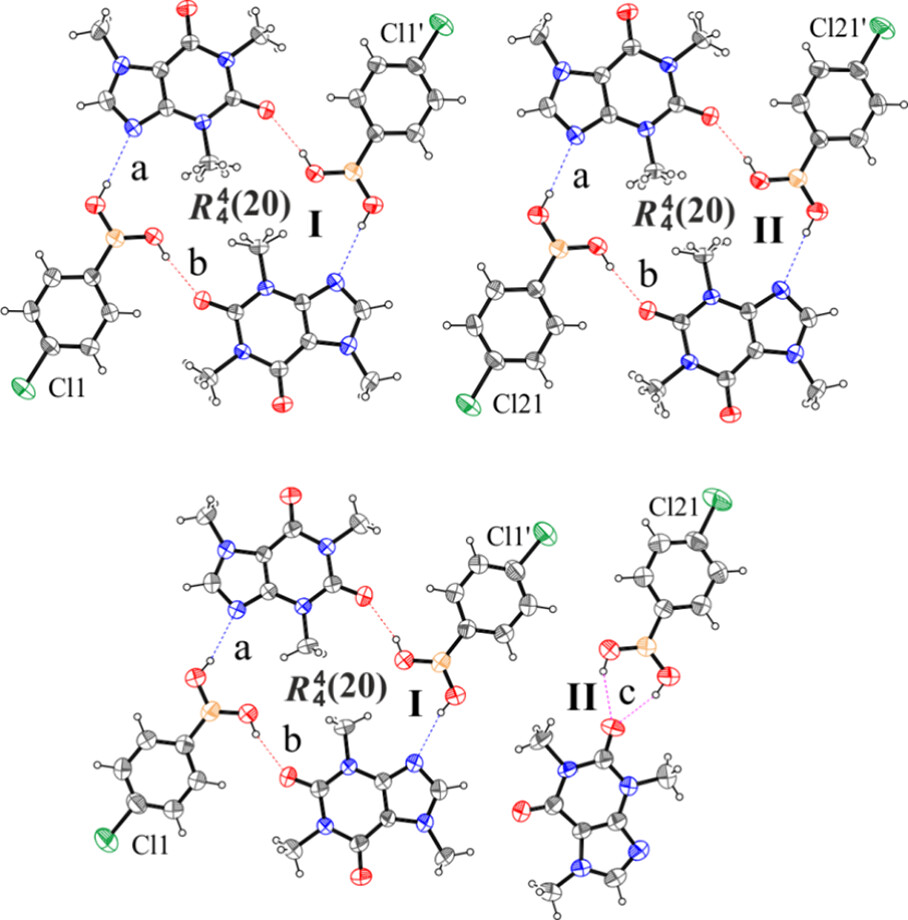
The tools that are part of the CSD-Materials and CSD-Particle suites were hence used to study and compare the structures of the two polymorphs.
The Mogul geometry check identified that the bidentate H-bonded motif in polymorph B involves a B‒C bond that is unusually short, while no alerts were found in polymorph A. From the calculation of the energetic stability, it was seen that the bidentate H-bonded motif was the strongest synthon in polymorph B, indicating that this motif might be more energetically favourable for the system.
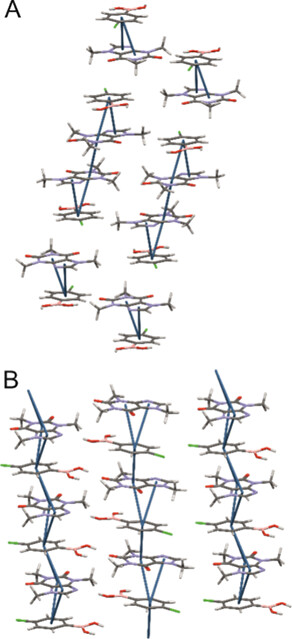
The four strongest synthons in polymorph A were instead identified with the four aromatic interactions found in the system. The new Aromatics Analyzer 2 (available to the public shortly) allowed the scientists to visualize and analyse dimeric and tetrameric stacks in polymorph A, and the infinite stacks in polymorph B (Figure 5). Analysing the structures in the CSD, it was confirmed that it is typical to see both oligomeric and polymeric motifs in cocrystals of caffeine.
Finally, the study of the morphologies was performed with the software VisualHabit, which allows the user to calculate the interactions along specific crystallographic directions, and hence to predict the crystal morphologies.
Polymorph A and B were predicted to be respectively a plate and a needle, in agreement with the crystal morphology dimensions recorded from the single-crystal X-ray diffraction (SCXRD) data.
Additionally, the investigation of the fastest growing facets was performed. It was concluded that while the stability of polymorph A relies on aromatic stacking, its particle shape is strongly dictated by the directionality of the hydrogen bonds. On the other hand, polymorph B is stabilized by the bidentate H-bonded motif, but the aromatic stacking interactions dictate its direction of growth.
This analysis showed how intermolecular bonds and different motifs impacted the packing energetics and morphology of the two systems.
Conclusions
In conclusion, a variety of tools developed by the CCDC were used to perform the study, design and comparison of two new cocrystal systems constructed with caffeine and 4-chlorophenylboronic acid.
A thorough analysis of the two components was performed, and the cocrystal design and synthesis were presented. Several structural features of the two new polymorphs were investigated, ranging from their supramolecular motifs, usual and unusual bond lengths, system energetics and morphology.
Next Steps
The paper was published as part of Crystal Growth & Design virtual special issue “Legacy and Future Impact of the Cambridge Structural Database: A Tribute to Olga Kennard” and can be accessed here: Cryst. Growth Des. 2024, 24, 12, 5159–5170.
To discuss further and/or request a demo with one of our scientists, please contact us via this form or .