Desolvation Processes in Solvates of Niclosamide
This blog highlights the work published in Molecular Pharmaceutics by Jen E. Mann and co-workers on the analysis of the desolvation processes for the antiparasitic drug niclosamide. Read the full article.
Introduction
Niclosamide (NCL) is a drug that is used as an intestinal anthelmintic, and that has recently shown the potential to be repurposed as an antiviral, antibacterial, and for cancer therapeutics.
As with many other APIs, NCL crystallizes in a variety of crystalline forms, including polymorphs, hydrates and solvates. Although most of the marketed APIs are free from any solvents, solvates play a fundamental role in the development of a drug by allowing crystalline forms, that would not be accessible from the standard solution growth methods, to be obtained through intentional desolvation.
The desolvation products are not easy to predict: the solvent loss can lead to the formation of an isomorphous desolvate, a crystalline structure rearrangement and hence formation of a different lattice, or to the collapse of the pores and formation of an amorphous phase. Time-resolved structural studies are therefore important to detect intermediate phases, and improve understanding of the solid-state transformations that lead to the final product.
In the work herein presented, the scientists used time-resolved synchrotron powder X-ray diffraction (sPXRD) and thermogravimetric kinetic studies to investigate the induced desolvation process for the NCL monosolvates with methanol (SMeOH) and acetonitrile (SACN).
Results and Discussion
Similarly to the monohydrate crystalline form of NCL (HA), the molecules of NCL in the structures of SMeOH and SACN assemble into π-stacks along the a-axis, forming one-dimensional channels along the same direction (Figure 1). It is important to notice that despite this, none of the three structures are isostructural, and so they exhibit three unique channel solvate topologies.
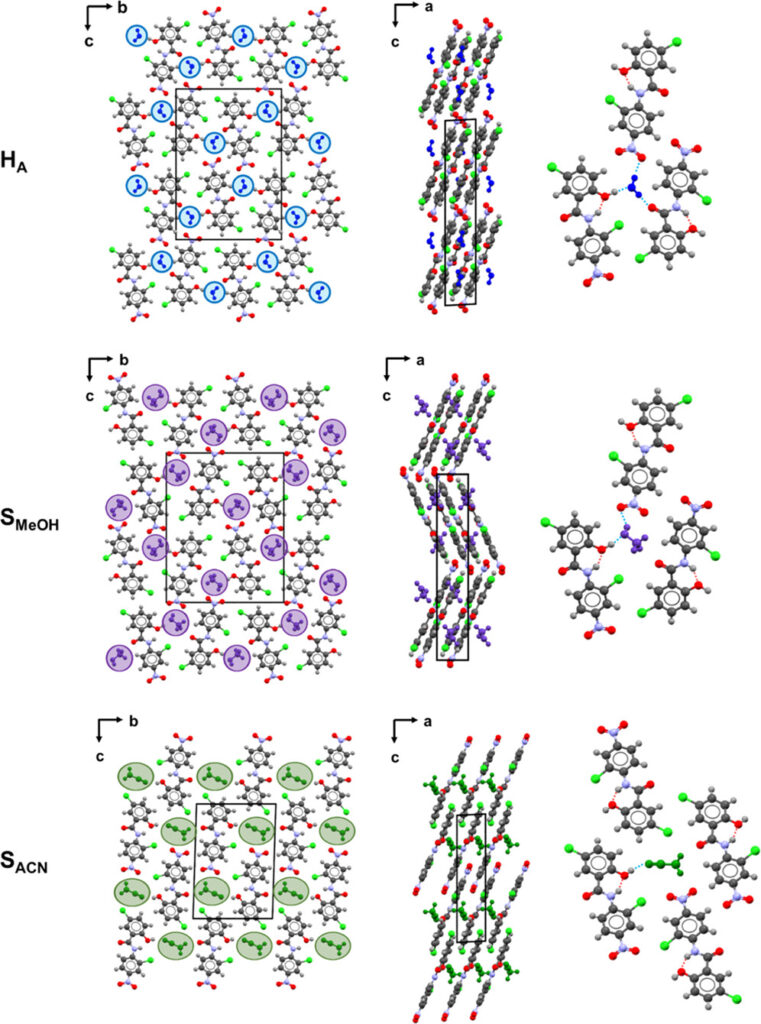
In this work, the scientists investigated the desolvation mechanism for the NCL monosolvates with methanol (SMeOH) and acetonitrile (SACN), aiming to identify the intermediate crystalline forms (if present) and to understand if the solvent loss and the lattice changes (typically present) occur simultaneously or not.
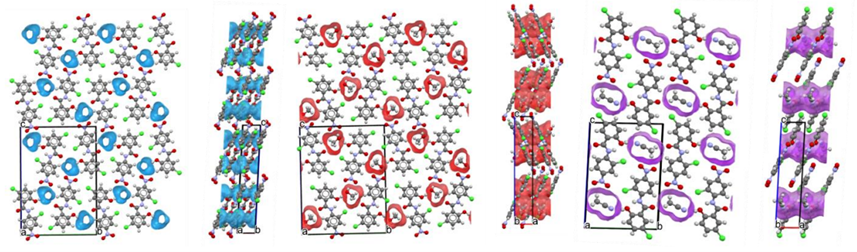
The Solvent Volumes
The desolvation study started with the estimation of the solvent volumes using the Solvate Analyser component in Mercury (Figure 2). Using a probe radius of 1.0 Å and a grid spacing of 0.3 Å, the solvent volumes obtained as a fraction of the unit cell volume were 7.3% in HA, 12.0% in SMeOH, and 17.8% in SACN.
Dehydration of HA
The team had previously reported in the literature the dehydration process studies of HA [2]. In that work, they identified that upon heating and solvent loss, HA formed an isomorphous anhydrate that was stable up until the temperature of 150 °C. At higher temperatures, the structure of HA changed and a polymorph with higher density (anhydrate Form 1, F1) formed.
Desolvation of SMeOH and SACN
The thermal analysis for SMeOH and SACN was performed with Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA). The DSC analysis showed that the endothermic transition for the desolvation occurred at the temperature of Tmax-MeOH = 77.4 ± 1.1 °C for SMeOH, and Tmax-ACN = 76.6 ± 1.3 °C for SACN. From the TGA data it was possible to see that the greatest weight loss in SMeOH happened between 58 °C and 75 °C (7.7 ± 0.1 wt %), while in SACN this interval was between 55 °C and 74 °C (9.5 ± 0.1 wt %).
Upon investigation of the desolvation process with time-resolved in situ sPXRD, the team could confirm the sample phase purity at the beginning of the experiment for SMeOH and could follow the structural changes upon heating. Only small changes were detected in the positions and intensities of the peaks up until the temperature of ~58 °C, which can be explained by the thermal expansion and partial solvent loss. A new peak with low intensity appeared at ~44 °C, which did not correspond to any intermediate/anhydrate known forms, and then disappeared quickly. Finally, at the temperature of ~58 °C, the peaks corresponding to the anhydrate known form F1 started appearing, with increasing intensity upon temperature increase. At 71 °C, the peaks for SMeOH disappeared completely and only the ones from F1 were present.
The same analysis was performed on SACN. Similarly to that seen for SMeOH, the sample phase purity was confirmed at the beginning of the experiment, and only small changes were detected in the positions and intensities of the peaks up to 65 °C. Two new transient peaks with low intensity appeared at ~33 °C which could not be assigned, and the peaks of F1 were first observed at 65 °C, becoming the only phase present at 76 °C.
Comparison of Desolvation in HA, SMeOH and SACN
The desolvation of HA, SMeOH and SACN formed the same product: the high-density anhydrate Form 1, F1. For HA, the TGA showed the water loss was complete at ~85 °C, but the sPXRD showed that the hydrate lattice was stable up to 150 °C. On the contrary, in SMeOH and SACN the desolvation temperatures and the temperature at which F1 was first observed were closer. This allowed the researchers to suggest that the solvate lattices of SMeOH and SACN had a substoichiometric solvent content when F1 started forming.
Another interesting observation is that the similar onset temperatures for the weight loss seen in the TGA (Tonset = 58 °C for SMeOH and Tonset = 55 °C for HA and SACN) seemed to not be correlated with the very different boiling points of the solvents (water, 100 °C; methanol, 64.7 °C; acetonitrile, 81.6 °C). The different hydrogen-bonding environment and volatility of the solvents are instead more likely to influence the reaction kinetics.
The team initially also suggested that the reason why no stable isomorphous solvent-free intermediates were identified for SMeOH and SACN could be related to the fact that these forms might not be dense enough to be stable intermediates. A deeper analysis of this aspect revealed additional complexities with the mechanism of desolvation. The scientists looked at the thermal expansion of HA, SMeOH and SACN, and noticed that the unit cell volume in SMeOH and SACN expanded at a considerably faster rate compared to the one in HA. While the latter showed a smooth transition facilitated by anisotropic thermal expansion from HA to the stable isomorphous anhydrate form and finally to F1, SMeOH and SACN showed some sudden changes in their lattices upon heating. This could indicate that a critical point was reached, influencing the route of the desolvation process.
Conclusions
This work reports a thorough investigation of the desolvation mechanism for the NCL monosolvates with methanol (SMeOH) and acetonitrile (SACN), and a comparison with the dehydration mechanism of the monohydrate crystalline form (HA).
The team used the Solvate Analyser component in Mercury to estimate the solvent volumes, DSC to find the endothermic transition temperatures, TGA to obtain the values of weight loss, and time-resolved in situ sPXRD to follow the structural changes upon heating. Putting together all the results, the scientists confirmed the inability of SMeOH and SACN to form an isomorphous solvent-free intermediate seen in HA, and suggested that this was due to sudden structure changes.
Next Steps
To discuss further and/or request a demo with one of our scientists, please contact us via this form or .