Zinc
Zinc:

Image of a US one cent coin. The coin is an alloy of 97.5% Zinc and 2.5% Copper since 1982
Facts about Zinc:
- Zinc: Zinc is a bluish-white metal, brittle at ambient temperatures while malleable between 100 to 150°C
- Fun fact about Zinc: Zinc is an essential mineral that is naturally present in some food, taken as a dietary supplement and found in some cold remedies. Oysters contain more Zinc per serving than any other food.
- Chemical symbol: Zn
- Atomic number: 30
A crystal structure containing Zinc:
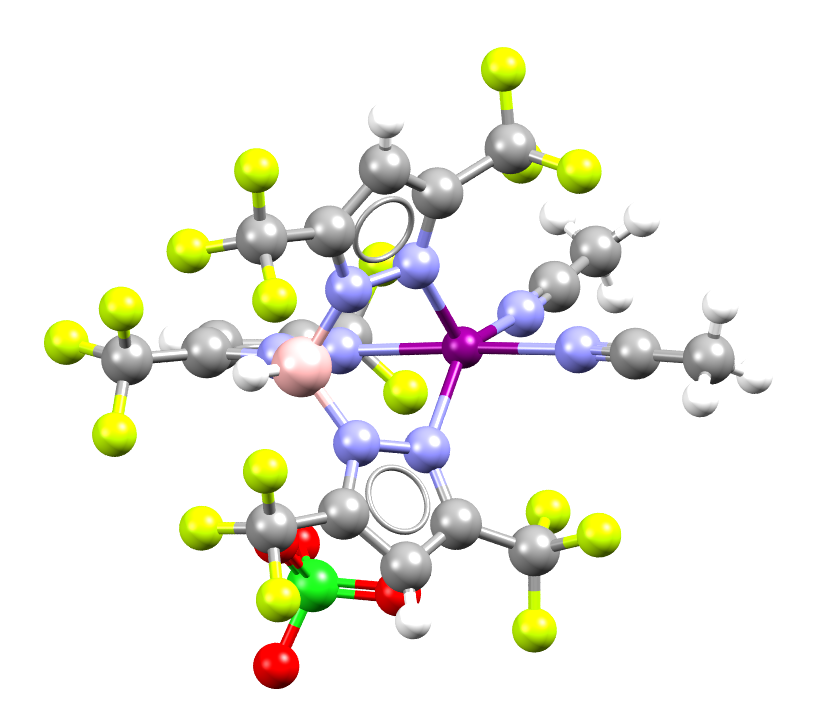
Image showing a Zinc(II) (shown in purple) complex that can catalyze C-H bond functionalization
Facts about this structure:
- Formula: C19 H10 B F18 N8 Zn +,Cl O4 –,C H2 Cl2
- Structure name: bis(Acetonitrile)-(hydrogen tris(3,5-bis(trifluoromethyl)pyrazolyl)borate-N,N’,N”)-zinc(ii) perchlorate
- Fun fact about the structure: The ligand bound to the Zinc atom belongs to a class called scorpionate ligands. The binding mode resembles a scorpion holding the metal with two pincers and the third donor site resembles a stinger.
- CSD refcode: JUWDOW (What’s this?)
- Associated publication: Naveen V. Kulkarni, Chandrakanta Dash, Naleen B. Jayaratna, Shawn G. Ridlen, Sarah Karbalaei Khani, Animesh Das, Xiaodi Kou, Muhammed Yousufuddin, Thomas R. Cundari, H. V. Rasika Dias, Inorganic Chemistry, 2015, 54, 11043, DOI: 10.1021/acs.inorgchem.5b02134
More about Zinc:
The discovery of pure metallic Zinc is credited to German chemist Andreas Marggraf but there are reports of previous isolation in India and by Swedish chemist Anton von Swab. Zinc is typically found in association with copper and lead in ores. Zinc forms alloys with other metals such as copper (forming brass), aluminium, and nickel. Zinc is used as an anti-corrosion agent by coating other metals such as iron with the element. Alloying copper with zinc can produce brass which is more ductile and stronger than copper while also conferring superior corrosion resistance on the resulting material. Another alloy of zinc and copper (with some other metals) is bronze, awarded as a medal to third place finishers in certain competitions like the Olympic games.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Zinc in a real crystal structure: