Uranium
Uranium:
Image of Becquerelite Uraninite with Uranophane crystals picture taken by Robert Matthew Lavinsky; Rob Lavinsky, iRocks.com – CC-BY-SA-3.0 [CC BY-SA (https://creativecommons.org/licenses/by-sa/3.0)]
Facts about Uranium:
- Uranium: Pure Uranium is a silvery white metal, extremely radioactive and extremely dense.
- Fun fact about Uranium: It is the heaviest naturally occurring element in the universe. It’s so dense, that a cube of Uranium (10cm x 10cm x 10cm) would weigh 19kg. It’s so heavy, once depleted, Uranium is used as a counter balance for aeroplanes and ships.
- Chemical symbol: U
- Atomic number: 92
A crystal structure containing Uranium:
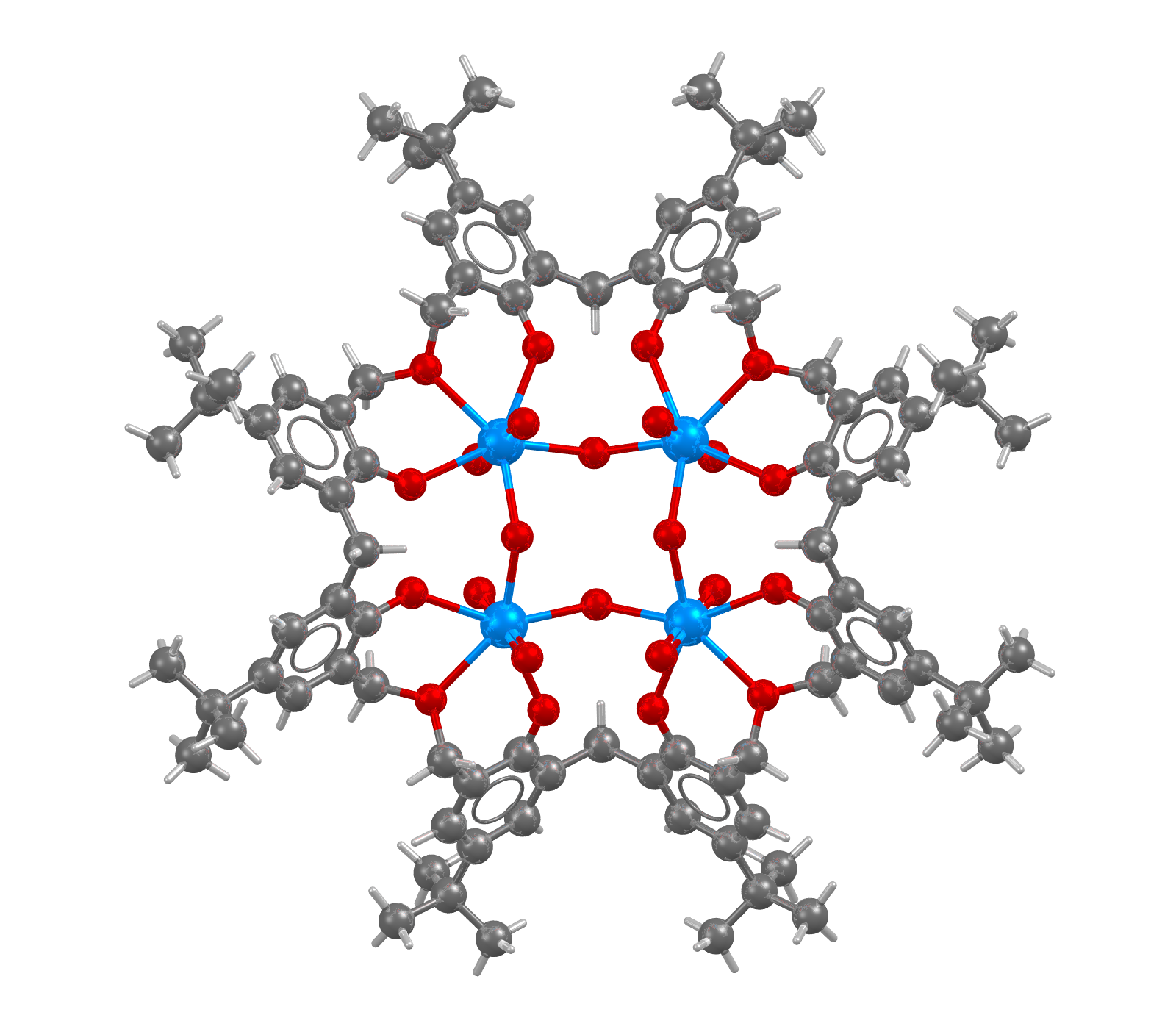
Image of a structure containing 4 Uranium atoms (in blue) in a calixarene with solvent molecules omitted for clarity
Facts about this structure:
- Formula: C92 H120 O24 U4,10(C H4 O)
- Structure name: (μ4-7,13,21,27,35,41,49,55-octa-t-butyl-2,3,16,17,30,31,44,45-octahomo-3,17,31,45-tetraoxa-57,58,59,60,61,62,63,64-octahydroxycalix[8]arene)-tetrakis(μ2-oxo)-octa-oxo-tetra-uranium methanol solvate
- Fun fact about the structure: This tetranuclear complex is highly symmetric.
- CSD refcode: AREYEB (What’s this?)
- Associated publication: P.Thuery, B.Masci, Polyhedron, 2003, 22, 3499, DOI: 10.1016/j.poly.2003.09.002
More about Uranium:
‘Yellowcake’ sounds nice, right? Be warned the cake is a lie, this is actually milled uranium oxide. I would highly advise you not to eat it. Uranium is commonly sold in this form before it is enriched. Uranium enrichment is a necessary process to create nuclear fuel. When Uranium is extracted from the crust it consists mainly of U-238 which is less radioactive. For Uranium to be useful it needs to be the isotope U-235 which is more radioactive, this is what the enrichment process does. From here Uranium can be either turned into fuel or used for nuclear weapons, if weapons are the goal a lot more U-235 is needed.
You don’t want to be eating ‘yellowcake’ but in reality, eating Uranium is normal to your body albeit in extremely miniscule quantities. Traces of Uranium can appear in rocks, soil and water. It gets absorbed by vegetables, sea creatures and animals which then ends up in our stomach. Thankfully everyone’s kidneys take the responsibility of removing it from our system.
Uranium is extremely dangerous. The bomb that was dropped on Hiroshima, contained 64kg of Uranium in it and only 1.38% of it underwent fission. That’s 0.88 kilograms of Uranium that caused the destruction of Hiroshima. Just imagine if all 64kg underwent fission.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Uranium in a real crystal structure:
