Samarium
Samarium:

Image of Samarium metal in its sublimed form.
Facts about Samarium:
- Samarium: Silvery, white metal, moderately hard, metallic in nature
- Fun fact about Samarium: Samarium is one of the rare earth elements that is used to make carbon arc lights. These lights are used in the motion picture and film industry for studio lighting and projector lights. The flints in gas lighters (e.g. Zippo lighters) are made up of material called Misch metal, where this metal contains about 1% of Samarium.
- Chemical symbol: Sm
- Atomic number: 62
A crystal structure containing Samarium:
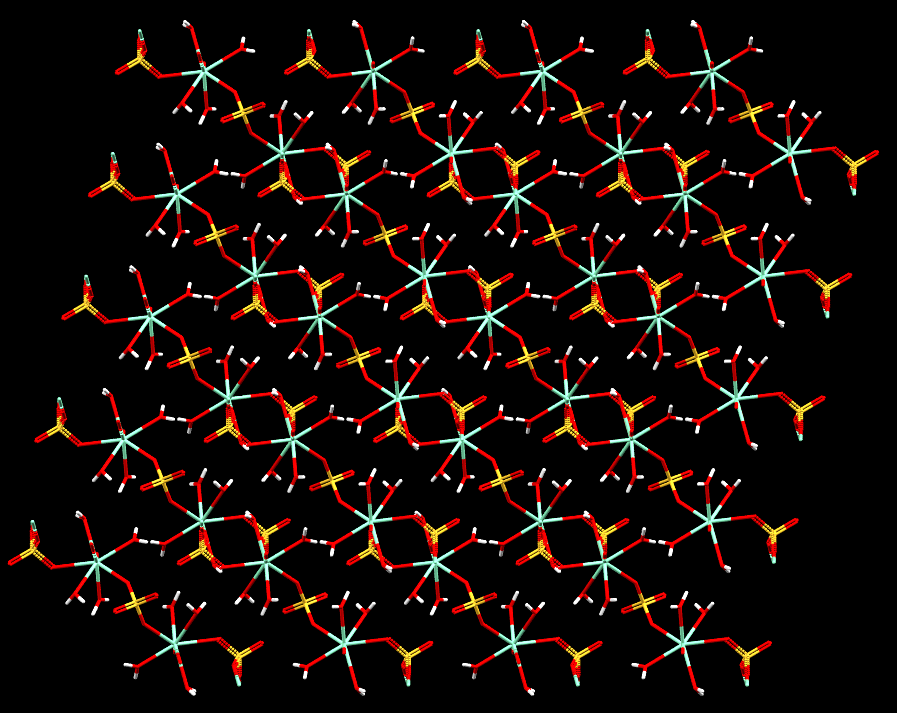
Image showing how Samarium, displayed as pale green atoms coordinated to water molecules, form polymer-like chains linked by sulphate molecules that appear layered in sheets in a 2-D network.
Facts about this structure:
- Formula: (H16 O20 S3 Sm2)n
- Structure name: catena-[tris(μ-sulfato)-octa-aqua-di-samarium]
- Fun fact about the structure: This structure was found to have excellent photoluminescent properties.
- CSD refcode: FAHDOK (What’s this?)
- Associated publication: Zhaoyan Deng, Fengying Bai, Yongheng Xing, Na Xing, Liting Xu, Open Journal of Inorganic Chemistry, 2013, 3, 76, DOI: 10.4236/ojic.2013.34011
More about Samarium:
Samarium was first observed by a Swiss chemist by the name of Jean Charles Galissard de Marignac, using spectroscopic methods, in 1853. A French chemist by the name of Paul-Émile Lecoq de Boisbaudran, was the first chemist to isolate Samarium from the mineral samarskite, in 1879. In modern day processes, Samarium is extracted and purified through an ion exchange process from monazite sand, which is a material rich in rare earth elements that can contains up to 2.8% Samarium. The primary use of Samarium is in the production of super magnets, where Samarium forms a compound with cobalt (SmCo5) which is a powerful permanent magnet with the highest resistance to demagnetization of any material known. In compound form, Samarium oxide (Sm2O3) is added to glass to absorb infrared radiation and this material also acts as a catalyst for the dehydration and dehydrogenation of ethanol (C2H6O).
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Samarium in real crystal structures: