Lead
Lead:

A piece of lead
Facts about Lead:
- Lead: When cut, lead is silvery with a hint of blue; but when exposed to air it becomes a dull grey, denser than most common materials, soft and malleable it is extremely stable
- Fun fact about Lead: If you ever make it to Venus you’ll see lead “snow” falling from the sky. The only downside is imminent death to due to Venus’ extremely high surface temperature of 469°C.
- Chemical symbol: Pb
- Atomic number: 82
A crystal structure containing Lead:
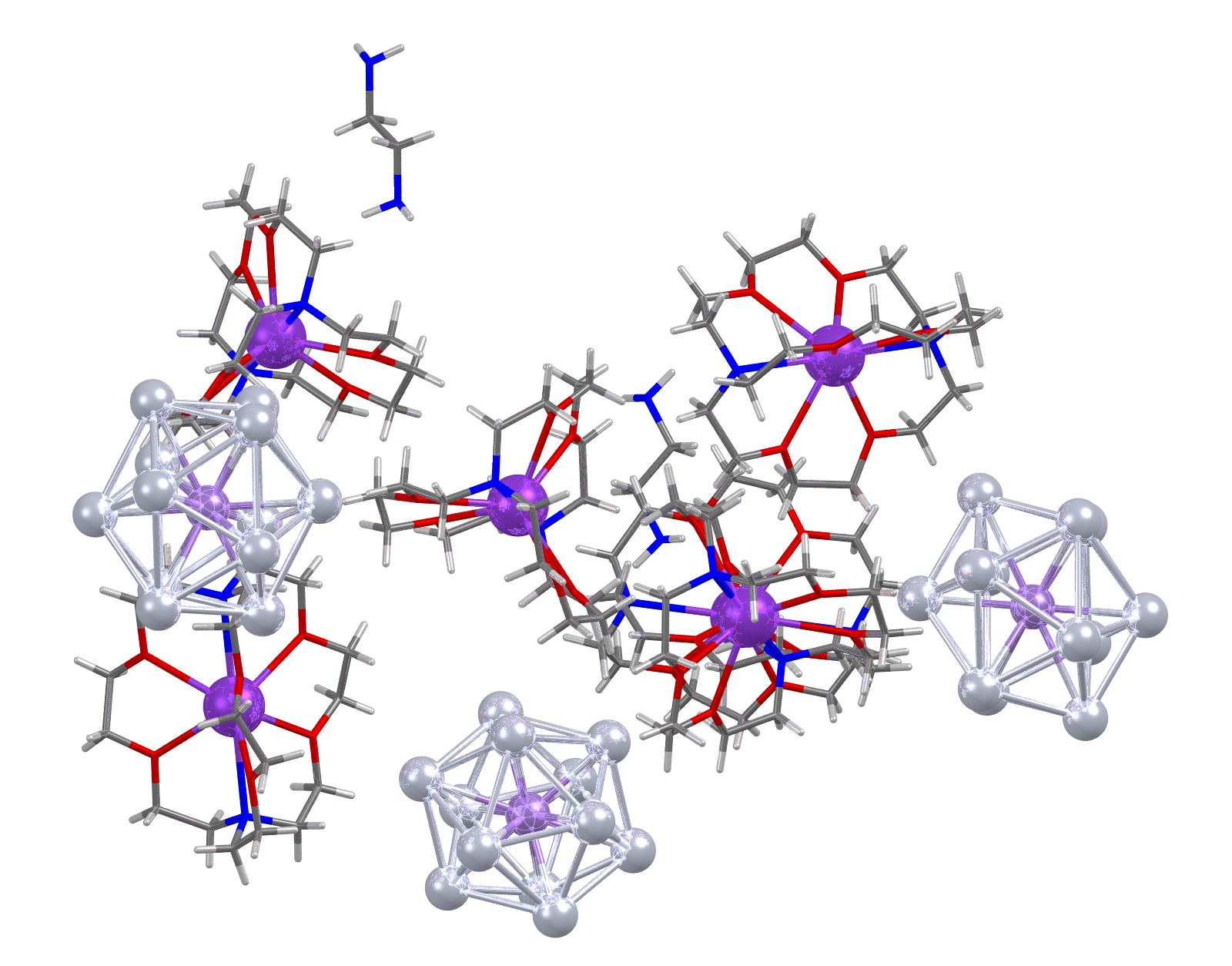
Image showing CSD Entry: ABONAI. Lead atoms shown as light grey spheres.
Facts about this structure:
- Formula: 3(C18 H36 K N2 O6 +),Mn Pb12 3-,C2 H8 N2
- Structure name: tris((4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo(8.8.8)hexacosane)-potassium) dodeca-lead-manganese ethylene-1,2-diamine solvate
- Fun fact about the structure: When reported this structure was the first example of an open-shell metal-centered deltahedral cluster anion, raising the possibility of extensive chemistry based on “magnetic superatoms” where a paramagnetic half-filled shell is trapped inside a diamagnetic cage.
- CSD refcode: ABONAI (What’s this?)
- Associated publication: Binbin Zhou, T.Kramer, A.L.Thompson, J.E.McGrady, J.M.Goicoechea, Inorganic Chemistry, 2011, 50, 8028., DOI: 10.1021/ic200329m
More about Lead:
Lead was one of the first metals to be discovered by humans. It was used in great amounts in the Roman Empire. The Ancient Romans made cookware, water pipes, wine jugs and coins. Romans boiled grapes, in Lead pots or Lead lined copper kettles, to make a variety of syrups. These syrups were used to sweeten and preserve wine and fruits. Each sip of wine and each bite of preserved fruit took the Ancient Romans closer to the Empire’s fall. Over half of the Lead produced is used in lead-acid car batteries. Most of the Lead produced nowadays comes from recycled batteries. In the 18th century, both men and women used powdered Lead to attain a fashionable ghostly complexion, even after the known toxicity of the substance. This lead to; eye inflammations, tooth rotting, baldness, and ultimately, death. Long term use caused the skin to blacken, creating a loop where wearers applied more and more each time until their demise. It has been reported that Queen Elizabeth I had a full inch of Lead makeup on her face when she died.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Lead in real crystal structures: