Iron
Iron:

Image shows a cylinder of pure iron, weighing 16 g and measuring 1 cm in diameter
Facts about Iron:
- Iron: A smooth iron surface are have a silver/gray colour. Iron readily reacts with oxygen and water to give brown/black hydrated iron oxides, known as rust. The rust flakes off which exposes fresh iron for corrosion.
- Fun fact about Iron: The most well known use of iron is probably as a catalyst in the Haber-Bosch process for the production of ammonia in World War 1.
- Chemical symbol: Fe
- Atomic number: 26
A crystal structure containing Iron:
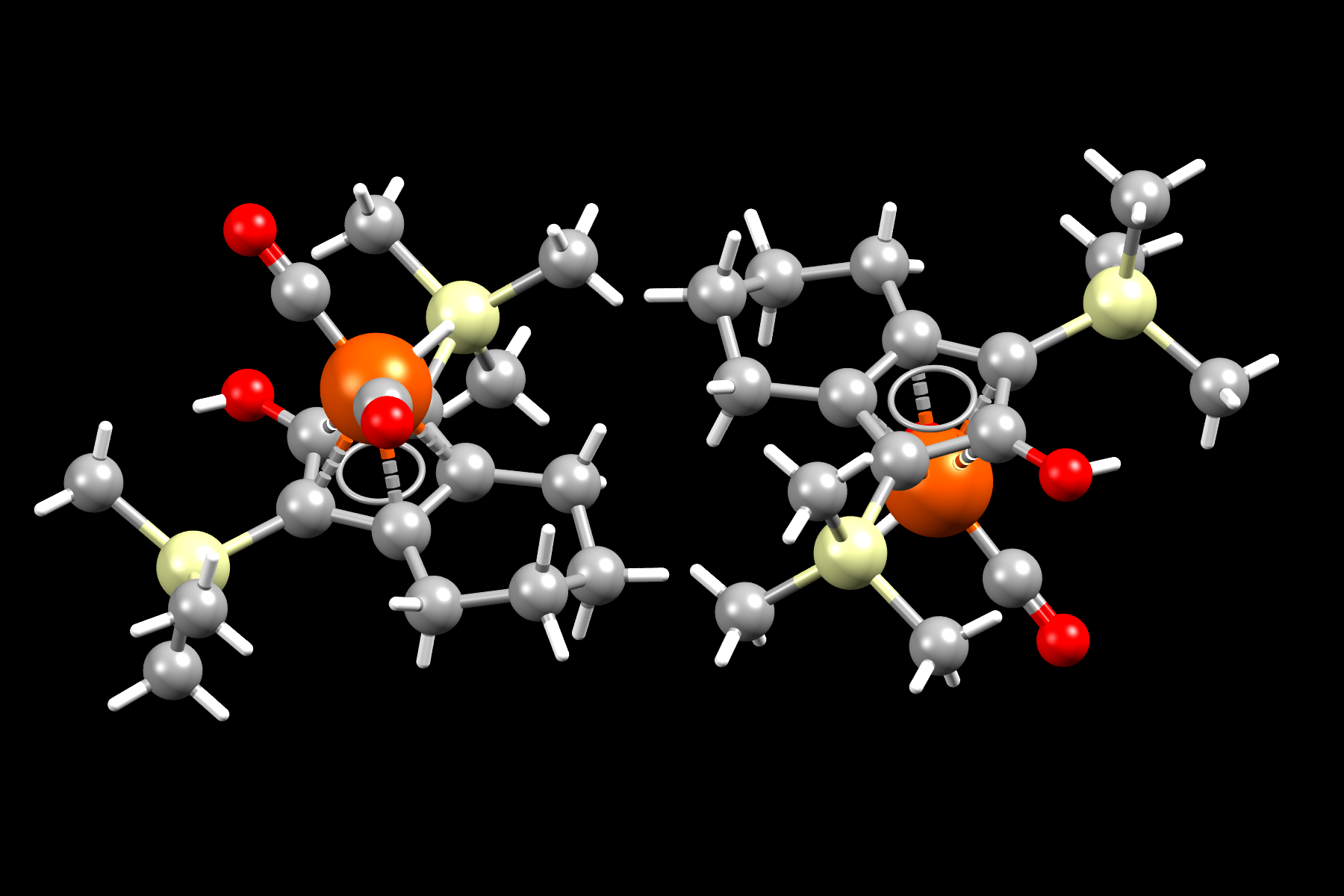
Image shows molecular representation of Knolker complex. Iron, oxygen, silicon, carbon atoms shown as orange, red, yellow and grey spheres respectively. Hydrogen atoms shown as white capped sticks.
Facts about this structure:
- Formula: C17 H28 Fe O3 Si2
- Structure name: Dicarbonyl-(η5-1,3-bis(trimethylsilyl)-4,5,6,7-tetrahydroinden-2-ol)-hydrido-iron
- Fun fact about the structure: This is the Knolker complex, a catalyst used for transfer hydrogenation. It is related to Shvo’s complex an organoruthenium catalyst also used for hydrogenation.
- CSD refcode: QAFJEM (What’s this?)
- Associated publication: H.-J.Knolker, E.Baum, H.Goesmann, R.Klauss, Angewandte Chemie, International Edition, 1999, 38, 2064, DOI: 10.1002/(SICI)1521-3773(19990712)38:13/14<2064::AID-ANIE2064>3.0.CO;2-W
More about Iron:
Iron is a transition metal, so called as it is placed in the first transition series and group 8 in the periodic table. Iron shows the characteristic chemical properties of transition metals, such as variable oxidation states and coloured complexes. Iron is the most common element, by mass, on Earth and it forms much of the Earth’s inner and outer core. It is the fourth most common element in the Earth’s crust. Iron’s chemical symbol is from the Latin “ferrum”. Iron has been used for millennia; beads made from iron in 3500 BC have been discovered in Egypt and China first produced cast iron in 5th century BC. Iron chloride and bromide complexes have long been used as catalysts in electrophilic aromatic substitution reactions. Iron complexes with organic ligands can be used as environmentally friendly catalysts such as the ammonia-borane dehydrogenation process. Iron is also magnetic which you may know from cool experiments in school using iron fillings to see the shape of magnetic fields!
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Iron in real crystal structures: