Gallium
Gallium:

The 2014 Nobel Prize in Physics was awarded to the discoverers of blue LED lights made from gallium.
Facts about Gallium:
- Gallium: A soft silver-grey metal
- Fun fact about Gallium: Gallium melts in your hand! Gallium melts at 29.76 deg.C, so will melt if you pick it up and freeze when you put it down again. (You: 36 deg.C; Room: 20-25 deg.C)
- Chemical symbol: Ga
- Atomic number: 31
A crystal structure containing Gallium:
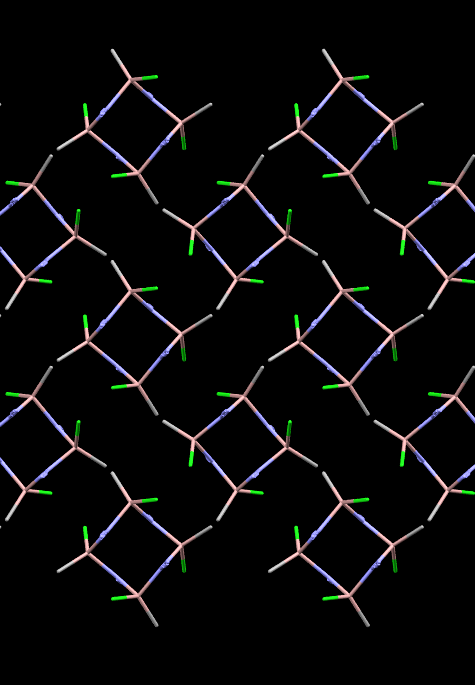
Image showing an azidogallium crystal with a ring of alternate Ga and N atoms.
Facts about this structure:
- Formula: C4 H12 Cl4 Ga4 N12
- Structure name: Cyclo-tetrakis((μ2-azido-N,N)-chloro-methyl-gallium)
- Fun fact about the structure: Gallium nitride is needed to make semiconductors, but explodes if you scratch it(!) This azidogallium structure is safer to handle and can be used to make devices without blowing up the factory.
- CSD refcode: EBIDIC (What’s this?)
- Associated publication: J. Kouvetakis, J. McMurran, Cory Steffek, T.L. Groy, John L. Hubbard, Inorganic Chemistry, 2000, 39, 3805, DOI: 10.1021/ic000053h
More about Gallium:
Gallium has a special place in the Periodic Table. When Mendeleev constructed his first Table, he left gaps and even predicted properties of some missing elements. Gallium was subsequently discovered in 1871 and found to have the properties he had predicted, thus cementing the reputation of the Table. The discoverer, a French chemist, Paul Lecoq de Boisbaudran named the new element after his native country, Gaul (France), but others alleged he had named it after himself, as Le coq means rooster and the Latin for rooster is gallus. He denied this. Gallium is unusual. It will melt in your hand (at 29.76 deg.C) but doesn’t boil until 2204 deg.C, giving it the greatest temperature range of any liquid element. It is very soft at room temperature – more like modelling clay than tough metal! Gallium has become increasingly useful this century as it is used in electronics. Gallium arsenide and nitride are used to make semiconductors and blue/violet LEDs, so are found in modern devices such as smartphones and Blu-Ray players. Sources: Wikipedia, Brittanica, Livescience
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Gallium in real crystal structures: