Europium
Europium:

Europium complexes are often used for phosphorescence applications. For example, they are used as anti-counterfeiting phosphors in euro bills that are visible upon irradiation with ultraviolet light.
Facts about Europium:
- Europium: grey, soft metal that can be dented with a fingernail and that rapidly oxidizes in air and must be stored under oil.
- Fun fact about Europium: Many chemists know Eu(hfc)3 which is a Europium complex that allows them to figure out, using nuclear magnetic resonance spectrometry, how much of different mirror images (enantiomers) of a compound are in their sample. [4]
- Chemical symbol: Eu
- Atomic number: 63
A crystal structure containing Europium:
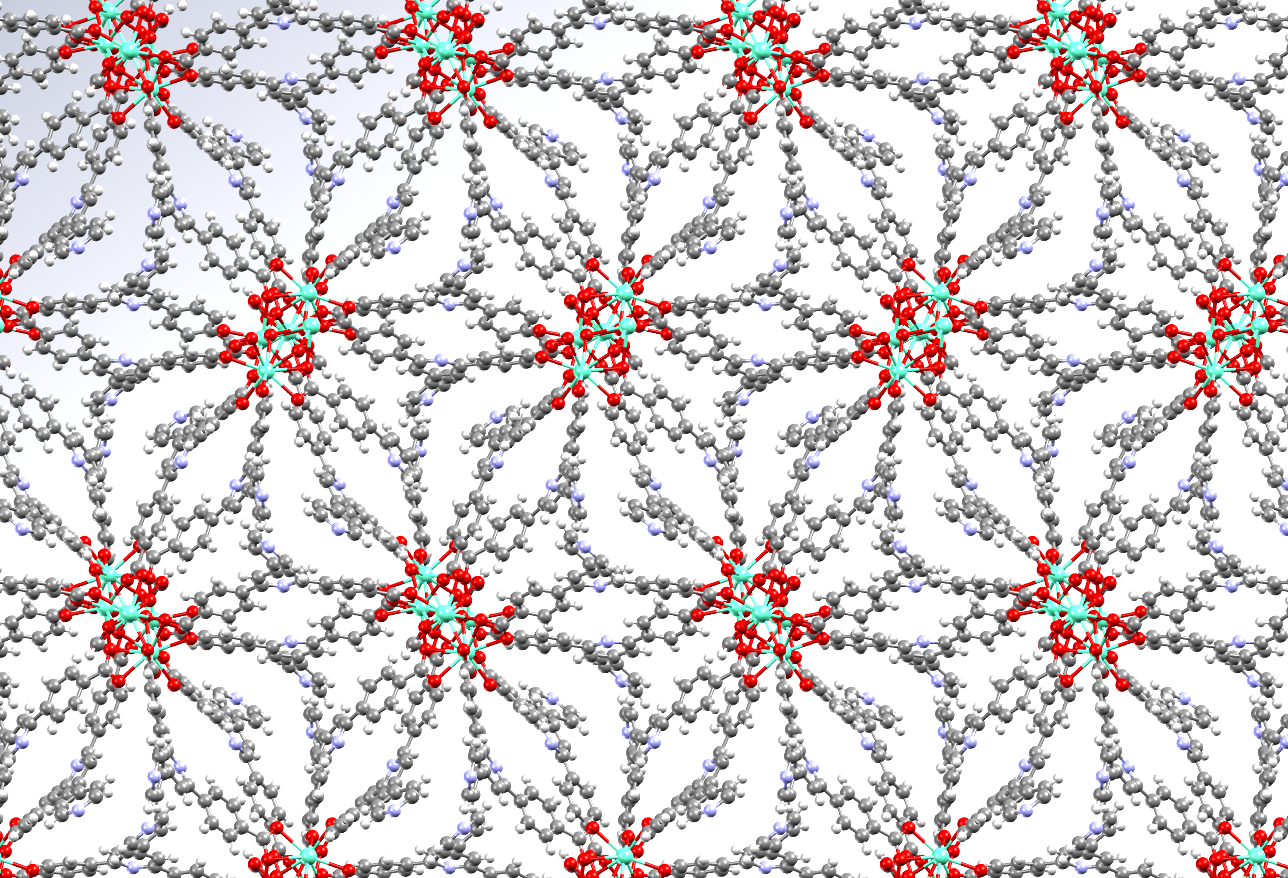
The image shows a Europium metal-organic framework in which the luminescence properties are used to build a multi-responsive sensor that can detect for example small organic molecules.
Facts about this structure:
- Formula: (C73 H47 Eu3 N6 O17)n
- Structure name: catena-[tris(μ-4,4′-(4,4′-bipyridine-2,6-diyl)dibenzoato)-(μ-formato)-bis(μ-hydroxo)-aqua-tri-europium unknown solvate]
- Fun fact about the structure: The framework indicates the adsorption of picric acid, an explosive, by reduction of its luminescence.
- CSD refcode: LORRUH (What’s this?)
- Associated publication: Xue-Zhi Song, Shu-Yan Song, Shu-Na Zhao, Zhao-Min Hao, Min Zhu, Xing Meng, Lan-Lan Wu, Hong-Jie Zhang, Advanced Functional Materials, 2014, 24, 4034, DOI: 10.1002/adfm.201303986
More about Europium:
Europium breaks the ranks. It is the most reactive rare-earth element and, in contrast to most other lanthanides, it also forms stable divalent compounds due to the stable half-filled f electron shell for Eu(II). Many of its applications are related to the f electrons which transitions lead to sharp lines in the luminescence spectrum, which can, for example, be used in colour TV screens or in anti-counterfeiting. [1] For three decades, Europium has been used in bio probes in immunoassays to detect the presence of a compound or biomolecule. Also, its discovery is connected to the lines in the emission spectrum. In 1901 Demarçay reported the isolation of a new element, for which he proposed the name Europium and which previously was observed as an anomalous red line in the emission spectrum of samarium. [2] Curiously, its stable divalent state leads to a geochemical phenomenon that is known as “europium anomaly” which describes that it is often found to be enriched or depleted in minerals compared to other rare earth elements. This can happen for example, when Eu(II) replaces Ca(II) in some minerals. [3]
References:
[1] Suyver, F.; Meijerink, A. Europium Beveiligt de Euro. Chemisch2Weekblad 2002, 98 (4), 12–13.
[2] Bünzli, J.-C. Europium in the Limelight. Nature Chem 2010, 2 (8), 696–696.
[3] Goldschmidt, V. M. Geochemistry; Oxford Univ. Press: London, 1958.
[4] Axt, M.; Alifantes, J.; Costa, V. E. U. Use of Chiral Lanthanide Shift Reagents in the Elucidation of NMR Signals from Enantiomeric Mixtures of Polycyclic Compounds †. J. Chem. Soc., Perkin Trans. 2 1999, No. 12, 2783–2788.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Europium in real crystal structures: