Cerium
Cerium:

Cerium makes sparks when struck and is the main component of the brittle ferrocerium alloy that is used in lighter flints. (Image source: Ricardo Liberato / CC BY-SA (https://creativecommons.org/licenses/by-sa/2.0)).
Facts about Cerium:
- Cerium: Cerium is a soft rare-earth metal, that can be cut with a knife, and that tarnishes when exposed to air as an oxide layer builds up.
- Fun fact about Cerium: Even though Cerium belongs to the rare-earth elements it is not that rare: It is nearly as abundant as copper and five times as abundant as lead.
- Chemical symbol: Ce
- Atomic number: 58
A crystal structure containing Cerium:
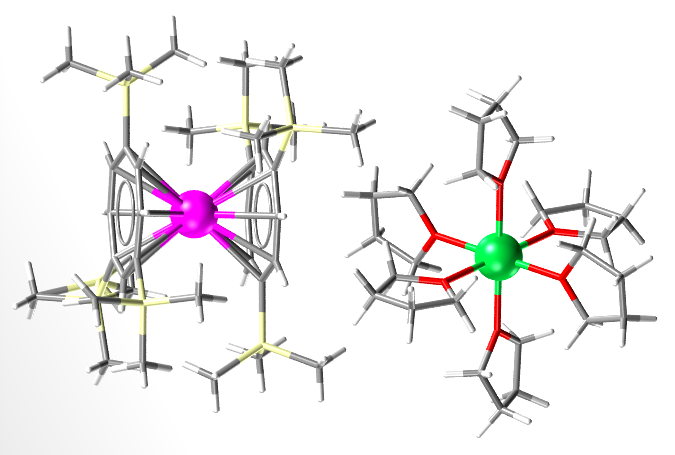
This structure is a variation of the Cerocene (a Cerium sandwich complex, about which electronic structure chemists have a lot of discussions). It is also the first organolanthanide complex with two different lanthanides. Cerium in magenta, Yb in green, Si in yellow, C in grey and H in white.
Facts about this structure:
- Formula: C24 H48 O6 Yb 2+,2(C34 H64 Ce Si6 –),C4 H8 O
- Structure name: hexakis(Tetrahydrofuran)-ytterbium bis(η8-1,3,6-tris(trimethylsilyl)cyclo-octatetraenyl)-cerium(iv) tetrahydrofuran solvate
- Fun fact about the structure: The researchers used the silyated Cerocence (i.e., the one with Me3Si groups on the aromatic rings) because Cerocene is too reactive and pyrophoric.
- CSD refcode: DAQFIK (What’s this?)
- Associated publication: U.Reissmann, L.Lameyer, D.Stalke, P.Poremba, F.T.Edelmann, Chemical Communications, 1999, 1865, DOI: 10.1039/a903954f
More about Cerium:
Cerium can make sparks when struck, but it is also used in “flammacerium” (cerium nitrate) as an antimicrobial agent for burns. [1] Cerium stands out from other rare-earth elements by not only being rather abundant, but also by being one that can be easily separated from a mixture of rare-earth elements (which are chemically quite similar). This is because it can be oxidized to cerium(iv)oxide (ceria) that can be readily filtered off from the other lanthanides in acidic solution. [2] Ceria is used for the polishing of precision optics as well as to decolorize glass and protect it against browning.
But we also find it as a catalyst in self-cleaning ovens, for the production of fuel or to help combust un-burnt fuel in the exhaust pipe of cars.
Ceria also shows a fascinating aspect about the chemistry of cerium: the oxidation state IV appears to be quite common but close inspection reveals that it is typically more something between III and IV.
Ceria, for example, has vacancies where oxygen atoms are missing and which makes it useful for the catalytic applications as it can cycle between storage and release of oxygen. [3]
References
[1] Ross, D.; Phipps, A.; Clarke, J. The Use of Cerium Nitrate-Silver Sulphadiazine as a Topical Burns Dressing. British Journal of Plastic Surgery 1993, 46 (7), 582–584.
[2] Reinhardt, K.; Winkler, H. Cerium Mischmetal, Cerium Alloys, and Cerium Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA, Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; p a06_139.
[3] Schelter, E. J. Cerium under the Lens. Nature Chem 2013, 5 (4), 348–348.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Cerium in real crystal structures: