“Hotspot mapping” accelerates early-phase drug design
CCDC, Exscientia, and Oxford University collaborate on an automated, quantitative method for informing the design of compound selectivity across protein families
Cambridge Crystallographic Data Centre (CCDC), Cambridge, UK— 2 March 2022 —The amount of structural data on protein drug targets continues to grow. However, successfully mining this data to form testable hypotheses that drive drug discovery can prove challenging. Selectivity for the target protein is a crucial property in the development of new therapeutics. In a recent paper in the Journal of Chemical Information and Modeling, authors from the CCDC, Exscientia, and Oxford University show how an automated process leveraging “ensemble hotspot maps” can identify key structural differences that contribute to the selectivity of a compound for one protein over another.
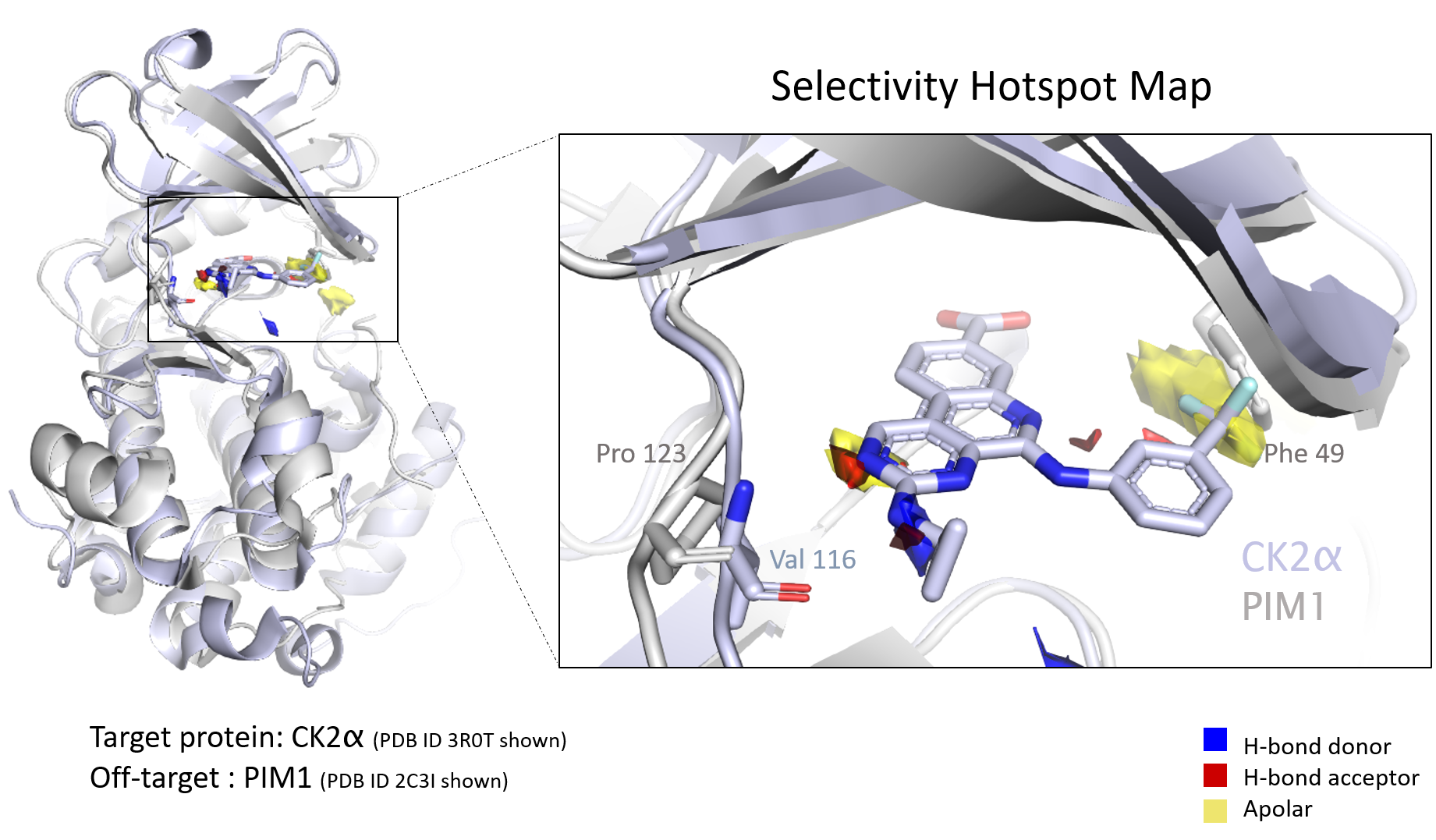
Hotspot maps use empirical data to assess protein binding sites to understand the druggability of the pocket, prioritize drug design, and spot differences in similar proteins that might drive compound selectivity.
The power of hotspot mapping to advance drug design
Hotspot mapping quantifies the propensity for compounds to use interactions in a preferred binding site—providing a 3D grid of data to help score and prioritize compounds. The power of this method lies in how it finds key interactions during early-phase drug discovery and then distils the information into easily interpretable results. Chris Radoux is Head of Structural Bioinformatics at Exscientia and a co-author on the paper.
“Adding hotspot maps early in a drug discovery project can provide a molecular blueprint using the protein structure alone,” says Radoux. “This can be used to help determine how druggable a given pocket of a target protein is and to prioritize fragment starting points for compound design. The highest scoring interactions can then be used to guide computational methods and algorithms.”
Hotspot maps drive cohesive drug design
This approach automates analysis across a protein family, as proteins in the same family often have similar binding sites. According to Mihaela D Smilova, co-author and postgraduate researcher at the Centre for Medicines Discovery at Oxford University, selectivity profiles in both the complete proteome—and within the target protein family—must be understood to develop safe and effective drugs. Interactions with unrelated target proteins can lead to unwanted side effects and toxicity, she says. However, effective drugs often leverage the benefits of “polypharmacology.”
“Introducing polypharmacology, or the ability to modulate multiple targets, may help to prevent the development of resistant disease phenotypes,” says Smilova. “Consequently, a successful drug candidate has a finely tuned selectivity profile within its target family—interacting with targets that positively impact the disease phenotype and avoiding interactions that lead to unwanted side effects.”
Using hotspot maps as inputs for computational workflows means researchers can rapidly explore the chemical space.
“This saves time by summarizing the information and presenting it in a way that is both interpretable by medicinal chemists and can be used in further computational analyses,” says Smilova.
Leveraging real-world, empirical data for reliability
The script used to generate the hotspot maps is a Python package called, “Hotspots API,” which leverages the data in the Cambridge Structural Database (CSD) via CCDC’s IsoStar library of interactions. The CSD is the world’s repository for small-molecule organic and metal-organic crystal structures—containing over 1.1 million structures from x-ray and neutron diffraction analyses. IsoStar is a web application that uses the CSD to generate thousands of interactive 3D scatterplots that show the probability of occurrence and spatial characteristics of interactions between pairs of chemical functional groups. Dr Jason Cole is a Senior Research Fellow at CCDC.
“Using CSD data for this type of analysis provides different insights from energy-calculation-based methods, as the interactions observed in the CSD are influenced by more than their strength,” says Cole.
Impacts of the study
Exscientia is a global leader in pharmatech, which sits at the interface of advanced AI application and complex drug discovery. They have implemented the hotspot mapping in-house within multiple drug discovery programs and use it to guide target validation and drug design. In addition, a research team at the University of Cambridge recently published in Nature how they used fragment hotspot mapping to identify structures that may assist in designing DNA-dependent protein kinase catalytic subunit inhibitors, which show potential as cancer therapeutics.
Learn more
Email us at to request a trial or demonstration.
Read the papers
Mihaela D. Smilova, Peter R. Curran, Chris J. Radoux, Frank von Delft, Jason C. Cole, Anthony R. Bradley, and Brian D. Marsden, J Chem Inf Model, 2022 62 (2), 284-294. DOI: 10.1021/acs.jcim.1c00823
Curran, P. R. et al. “Hotspots API: A Python Package for the Detection of Small Molecule Binding Hotspots and Application to Structure-Based Drug Design.” J. Chem. Inf. Model. 2020, 1911. DOI: doi.org/10.1021/acs.jcim.9b00996
Liang, S., Thomas, S.E., Chaplin, A.K. et al. “Structural insights into inhibitor regulation of the DNA repair protein DNA-PKcs.” Nature, 2022 601, 643–648. DOI: 10.1038/s41586-021-04274-9