Elusive chiral nitrogen compounds available in the Cambridge Structural Database
CAMBRIDGE 05 October 2021—CCDC’s Cambridge Structural Database (CSD) houses elusive chiral nitrogen compounds, especially important for drug discovery.
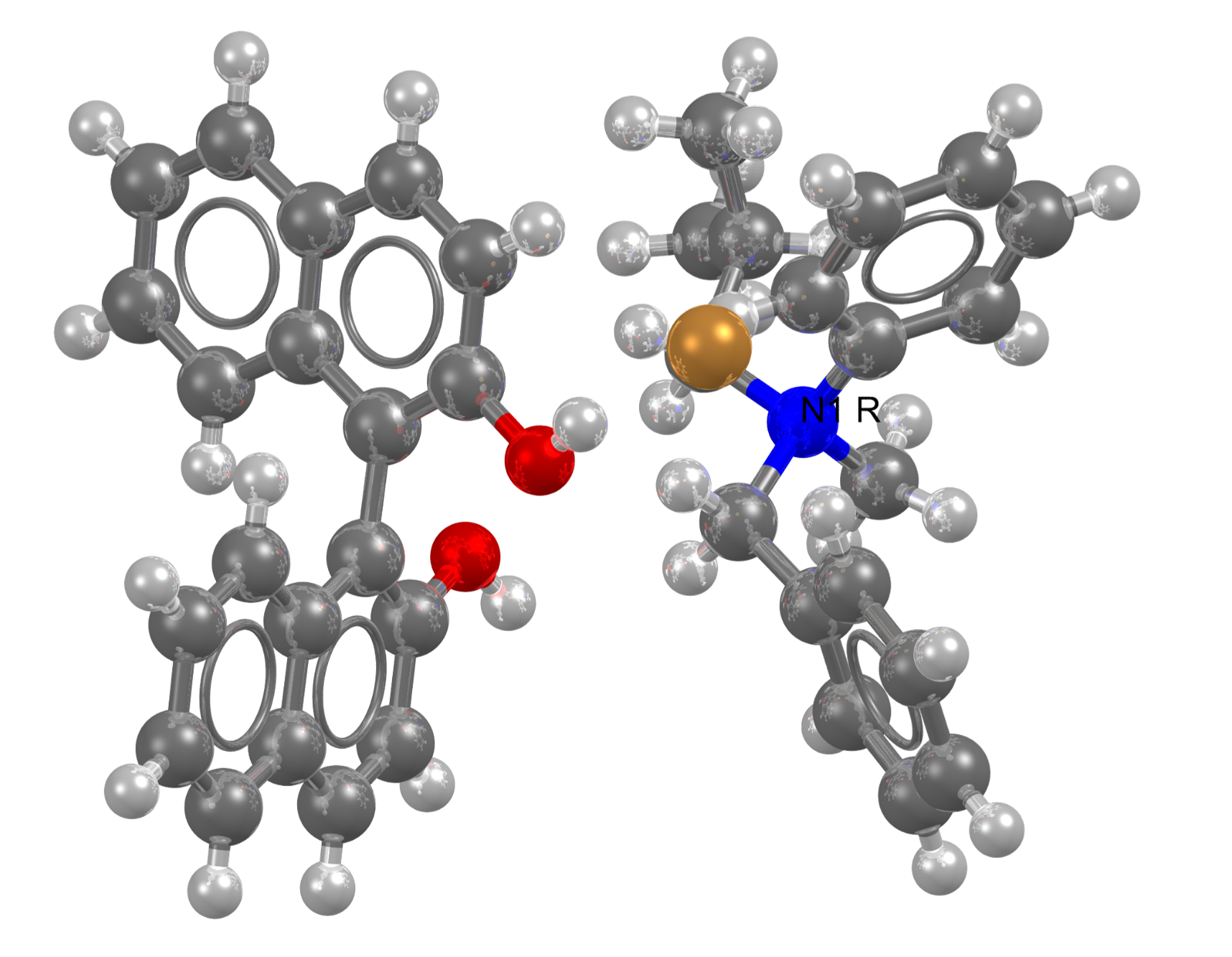
CSD Refcode: HADLOR (DOI: 10.5517/ccdc.csd.cc24ppdy)
To date, chemists have struggled to make compounds with chiral nitrogen centres, which are important for potential pharmaceuticals and other biologically active molecules. Recently, researchers at Durham University published in the journal Nature how to make the compounds with high specificity with a one-pot synthesis.
By adding a carbon fragment to an amine and binding the resulting ammonium to a common chiral compound, the authors produced chiral nitrogen compounds that locked the molecules into either the S or the R configuration. While they then knew they had different enantiomers, they didn’t know which ones until they crystalized them and solved the crystal structures.
View the structures in the CSD
These structures are now available in the Cambridge Structural Database (CSD), the world’s repository of over 1.1 million small-molecule organic and metal-organic crystal structures. CCDC deposition numbers:
- 1987042–1987058
- 1987061–1987068
- 1987165–1987180
- 2047299–2047303
In addition to having these hand-curated, annotated structures available in the CSD, the scientific community can also benefit from their contribution to CCDC’s statistical analysis suite. CCDC derives a variety of tools from the CSD, including:
- Mogul, a knowledge-based library of molecular geometry that provides precise information on preferred molecular geometries by enabling access to millions of chemically classified bond lengths, valence angles, acyclic torsion angles, and ring conformations.
- IsoStar, a web application that provides thousands of interactive 3D scatterplots that show the probability of occurrence and spatial characteristics of interactions between pairs of chemical functional groups.
- SuperStar, a knowledge-based pharmacophore generation and prediction of intermolecular interactions predicts “hot-spots” where a chosen interaction type is particularly favorable.
Read more
Read a summary of the article from C&EN.
Read the paper, “Enantioselective synthesis of ammonium cations.” Walsh, M.P., Phelps, J.M., Lennon, M.E. et al. Nature 597, 70–76 (2021).