Advancing pharmaceutical development through digital design
In a new study published in Crystal Growth & Design, researchers from CCDC, in collaboration with the University of Leeds, Britest Limited, Pfizer and GSK, introduced a new workflow that brings together existing and novel approaches in the assessment and prediction of particle properties important to the formulation and manufacture of pharmaceuticals.
The research aimed to provide resolutions at key decision points encountered during formulation design and manufacturing processes, to help reduce the high risk of failure associated with drug development.
Understanding particle and surface properties
It is well recognised in the pharmaceutical industry that very few Active Pharmaceutical Ingredients (APIs) actually end up on the market. Around 9 out of 10 drugs fail to make it to market; normally as a result of safety and efficacy issues, but also due to failures during the manufacturing process. With such high research and development costs coupled with this failure rate of over 90%, the return on investment is clearly a huge issue for the pharmaceutical industry.
With this in mind, solid form scientists from academia and some of the largest pharmaceutical companies in the world collaborated on a study proposing the significance of computational techniques to provide insight at earlier stages of development and manufacturing to maximise productivity and reduce attrition in downstream processes.
Despite a well-developed understanding of solid form properties, interpretation of the relationship between particle and surface properties and manufacturability is less established. The authors in this study proposed that by applying wider molecular modelling methodologies to inform the prediction of properties, such as particle cohesivity, morphology and surface energy, a deeper understanding could be gained into the problematic particle behaviours which ultimately lead to bottlenecks later in the manufacturing process.
The research was carried out by several partners of the ADDoPT project which aims to bring the concept of digital design to the formulation design and manufacturing stages of the pharmaceutical supply chain.
Applying big data to drive valuable insight
This work follows on from research published in 2011, whereby the authors applied the concept of solid form informatics to the 500,000th structure to enter the Cambridge Structural Database (CSD), the antiepileptic drug lamotrigine. This was able to inform the development of a solid form risk assessment protocol which is now widely adopted by the pharmaceutical industry.
The use of lamotrigine in this study was pertinent due to its established status as a ‘typical’ drug, with relatively well-known properties. The drug molecule exhibits low aqueous solubility, is often prone to capping upon tableting due to low compressibility of the API, and has poor powder flow properties, characteristics that can pose significant challenges within manufacturing processes.
In this latest research, the authors again used lamotrigine as a model drug, this time applying data from the one million structures in the CSD to assess its solid form in addition to exploring its particle properties.
They move lamotrigine through a proposed workflow, comparing insights gained at each step to enable a thorough understanding of its particle properties, which could be used to inform key decisions along the pharmaceutical manufacturing process, demonstrating the potential to streamline formulation design and manufacturing and ultimately deliver drugs to market faster.
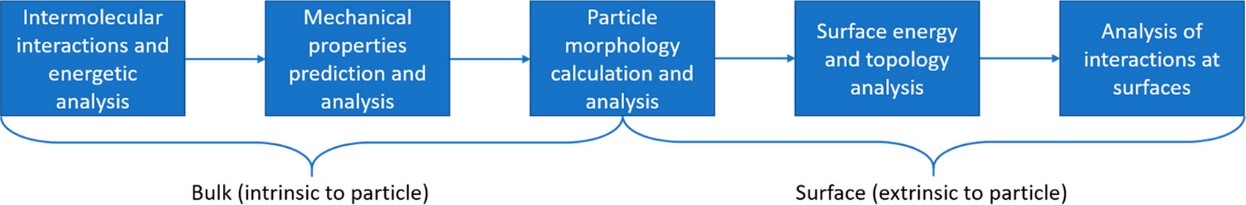
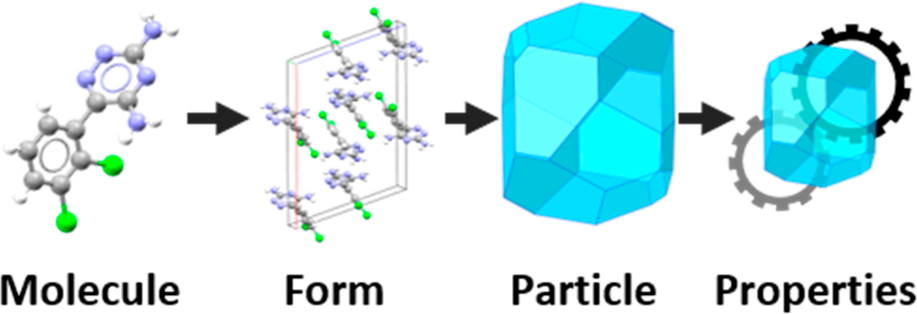
Proposed particle informatics workflow (source: Cryst.Growth Des.2019)
By analysing the intermolecular interactions, mechanical properties, surface energy and morphology of lamotrigine, the authors were able to rationalise issues apparent in the formulation and manufacturing of this drug.
The benefits for the pharmaceutical industry of adopting Particle Informatics into their formulation and manufacturing processes is clear. Not only does it have the potential to help avoid costly failures, it could also be beneficial to successfully deliver drugs to market more quickly.
The full article is published in Crystal Growth Design 2019, https://doi.org/10.1021/acs.cgd.9b00654.
Want to receive more news like this? Subscribe to the CCDC newsletter.