Q&A with Dr Eugenia Peresypkina and Dr Alexander Virovets: What are Cyclo-Pn ligand complexes and what could they mean for supramolecular chemistry?
Cyclo-Pn ligands are carbon-free analogues to aromatic organic ligands. They have lone electron pairs, which are reactive towards Lewis acidic transition metals to give various coordination. According to a recent review article co-authored by researchers at the University of Regensburg, these are a few reasons cyclo-Pn ligand complexes are so important to the field of organometallic chemistry. In this Q&A blog, we talk to two of the co-authors on that paper, Dr Peresypkina and Dr Virovets, about the team’s work, trends they found, and potential applications for the compounds.
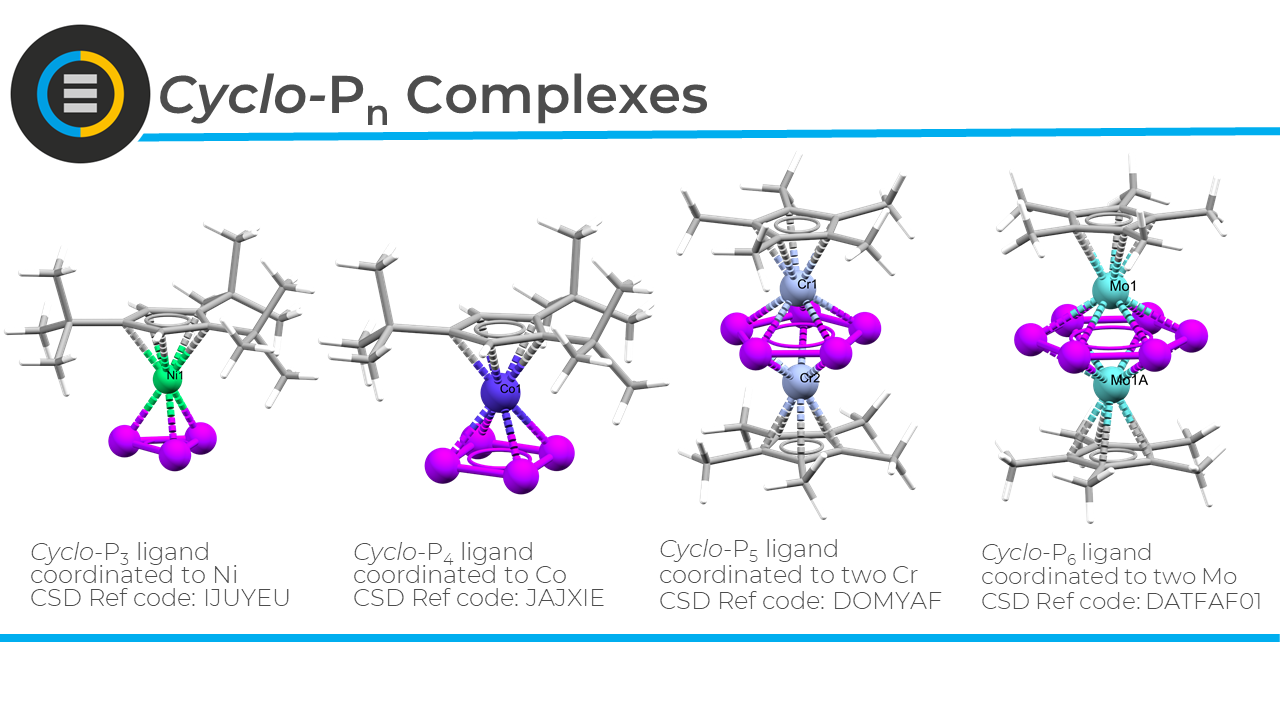
Polyphosphorus compounds serve as building blocks for supramolecular chemistry—meaning they can be combined via metal ions to form larger structures, like spheres, tubes, and coordination polymers. Importantly, they can also shed light on self-assembly processes in metallosupramolecular chemistry.
Dr Eugenia Peresypkina, Dr Alexander Virovets, and team leader Prof. Manfred Scheer co-authored Organometallic polyphosphorus complexes as diversified building blocks in coordination chemistry, a recent paper on the topic that focuses on the usage of the cyclo-Pn complexes as building blocks.
The paper says coordination polymers and supramolecules based on cyclo-Pn-ligand complexes show promise as “hosts for the selective encapsulation of guest molecules, guest separation, and reversible guest uptake.” Can you elaborate?
“The ability of a coordination polymer (CP) or a supramolecule to encapsulate a guest molecule depends on various factors,” Peresypkina said. “One of them is a possibility of specific, non-bonding, host-guest interactions that in turn depend on the presence of the specific donor or acceptor groups on the ‘internal’ surface of the host (the polymeric chain/framework or the supramolecule). For example, the presence of hydrogen bond donors facilitates the encapsulation of guest molecules possessing acceptor groups via the formation of host-guest hydrogen bonds. In this context, the cyclo-Pn building blocks can offer their phosphorus atoms for specific pnictogen-pnictogen and pnictogen-halogen host-guest interactions and thus facilitate encapsulation of the guest molecules with functionalities specific to such unique environment.”
“Back in 2013, we successfully encapsulated the metastable tetrahedral P4 and As4 molecules into the 1D-coordination polymer and the supramolecules both based on pentaphosphaferrocene (the cyclo-P5 building block), allowing the first determination of the molecular structure of the metastable, light-sensitive yellow arsenic, As4,” Peresypkina said. “To speak of perspectives, it would be interesting to find an application of molecular reactors for supramolecules based on cyclo-P5 ligands.”
Molecular reactors are miniature vessels for the assembly of reactants at the molecular level that change the nature of chemical transformations. For example, they can change product ratios or give products that would not readily form otherwise. (Chemistry. 2004 Jul 5; 10(13): 3120-8.)
What could new Pn-ligand discoveries mean for future chemistry?
“Polypnictogen complexes can be used as a synthetic platform for the synthesis of phosphines—especially asymmetric substituted phosphines mediated by pentaphosphaferrocene, Cp*Fe(cyclo-P5), to avoid the generation of PCl3 from white phosphorus and to start directly from P4 mediated by Cp*Fe(cyclo-P5),” Virovets said. “Here, talented chemists in our group have recently published a paper in Nature Communications (DOI: 10.1038/s41467-021-26002-7).”
Researchers want to avoid PCl3 because it’s toxic and reacts violently with water, releasing HCl as a gas. Therefore, anhydrous and oxygen-free environments are required.
Using CCDC’s ConQuest software for advanced 3D searching of Pn-ligand complexes and interactions
The team used CSD data and ConQuest for their analysis of cyclo-Pn-ligand complexes and interactions.
Can you explain how ConQuest helped you rationalize the coordination environments in these complexes?
“The idea of the usage of the polypnictogen complexes of M metal ions as building blocks for the metallosupramolecular chemistry is based on their ability to coordinate the additional metal cations to the phosphorus atoms,” Peresypkina said. “However, to design these complexes in a rational way, it is important to know how exactly the cyclo-Pn ligands bind different metal cations. Summarizing all known examples of coordination is a good way to understand these tendencies (preferable coordination modes, etc.).”
- How many phosphorus atoms coordinate to the metal cation(s) and in what coordination mode?
- How are the coordinated metal cations positioned relative to the plane of the cyclo-Pn ring and at what distances?
- How does the coordination mode depend on the number of phosphorus atoms and, for a given number, on the nature of the metal, metal cation(s), and additional ligands?
- How does the number of phosphorus atoms and the coordination mode of the cyclo-Pn ligand change the connectivity and dimensionality of the resulting bimetallic complex?
Virovets added, “Here, the CSD and ConQuest software provided us truly unique opportunities. ConQuest is a very convenient software integrated with the CSD—a comprehensive structural database. It allows summarizing the geometric characteristics of a molecular fragment of interest in a set of crystal structures and easily visualizing and analyzing the data. Without this tandem-like software, our review article would be neither so informative nor so complete. Now we are sure that no compound or crystal structure escaped our attention.”
Was there anything about the coordination environment you found particularly surprising?
“The cyclo-Pn-based building blocks can bind to a metal cation resulting in an end-on (σ) and side-on (π) coordination,” Peresypkina said. “We found that with the increasing of n from 3 to 5, the end-on coordination mode becomes more frequent. The geometric characteristics of these types of coordination are indistinguishable for the Pn complexes with n = 3 but become clearly different for n = 5. It is an interesting tendency that was not stated elsewhere and still needs better understanding. Moreover, for the most studied case of pentaphosphaferrocene (n = 5) the Ag+ cations, in contrast to Cu+ ones, show a clear tendency to prefer the side-on coordination mode. This information is crucial not only for the planning of the synthetic research in the metallosupramolecular chemistry, but it also gives a wider vision of the overall class of the compounds and shows perspective for theoretical investigations.”
What role can data mining play in advancing structural chemistry research?
“The single-crystal X-ray diffraction (SCXRD), being the most informative method of molecular structure determination, is routinely used as the first-resort analytical tool in synthetic chemistry,” Virovets said. “Often the use of the method is considered only by its straightforward outcome—the atomic connectivity of a desired compound.”
“But it can do more if we do not limit ourselves to determining the crystal structure of a novel compound but take a little pause and spend some time analyzing the whole structural information on the entire class of compounds—the coordination possibilities of metals and ligands—also obtained by others and collected in databases like the CSD,” he said.
Peresypkina added, “Generally, we should look not only at new features but also for repeating patterns or common features in the same or similar classes of compounds. Such crystallochemical analysis can help predict the way of coordination of metal cations to other building blocks and, therefore, to more rationally plan future research directed to novel metallosupramolecular architectures. We hope that our review will encourage more endeavours in this field and structural science will profit more from deductive approaches than it currently does.”
Dr Peresypkina and Dr Virovets would like to thank Prof. Manfred Scheer and all the students and postdoc researchers on his team for a great co-working experience, outstanding research, and motivating crystallographic challenges.
Read more
Read the full paper, Organometallic polyphosphorus complexes as diversified building blocks in coordination chemistry. (Coord. Chem. Rev., 2021, 446, 213995.)
Learn more about CCDC’s ConQuest software, the primary program for searching and retrieving information from the Cambridge Structural Database (CSD).
Read additional applications of CCDC tools, including case studies on optimizing metal-organic frameworks for VOC recapture and hydrogen storage and using the CSD to discover materials with electronic properties.
