ConQuest in Action: Introducing Intramolecular π-Interactions into Heteroleptic Complexes
Here we highlight how the use of ConQuest to search the Cambridge Structural Database (CSD) helped guiding the design and synthesis of new heteroleptic compounds – coordination complexes with more than one type of ligands.
Read the full article and find out more about the CCDC data, software, and services to advance structural science that were used in this work.
Why?
Heteroleptic copper(I) compounds can exhibit enhanced photoluminescence quantum yields (PLQY). This property is particularly interesting for applications in organic light emitting diodes (OLEDs) and light-emitting electrochemical cells (LECs).
In this work published in CrystEngComm, the scientists from the University of Basel reported the synthesis and characterization of six new heteroleptic copper(I) compounds. Beside performing solid-state photoluminescence studies, the team also analysed the crystal structures of the compounds and investigated the intramolecular interactions responsible for the enhanced emission.
How?
Species with formula [Cu(N^N)(P^P)]+, where N^N = aromatic diimine and P^P = chelating bis(phosphane) are known to be efficient LECs.
When the bis(phosphane) involved presents a wide bite angle (angle on a central atom between two bonds to a bidentate ligand), intramolecular π-stacking interactions can arise between the ligands, enhancing the emission and PLQY in these species. For this reason, the team chose to use xantphos and POP as P^P ligands.
The rationale for the design of the N^N ligands started instead with a search of the CSD using ConQuest. The team looked for salts of [Cu(6-Rbpy)(xantphos)]+ (with 6-Rbpy as N^N ligand and xantphos as P^P ligand) containing a range of R groups in N^N, and noticed that these groups prefer to point towards the xanthene unit of the P^P ligand (Figure 1. A) rather than away from it (Figure 1. B), forming intramolecular interligand interactions.
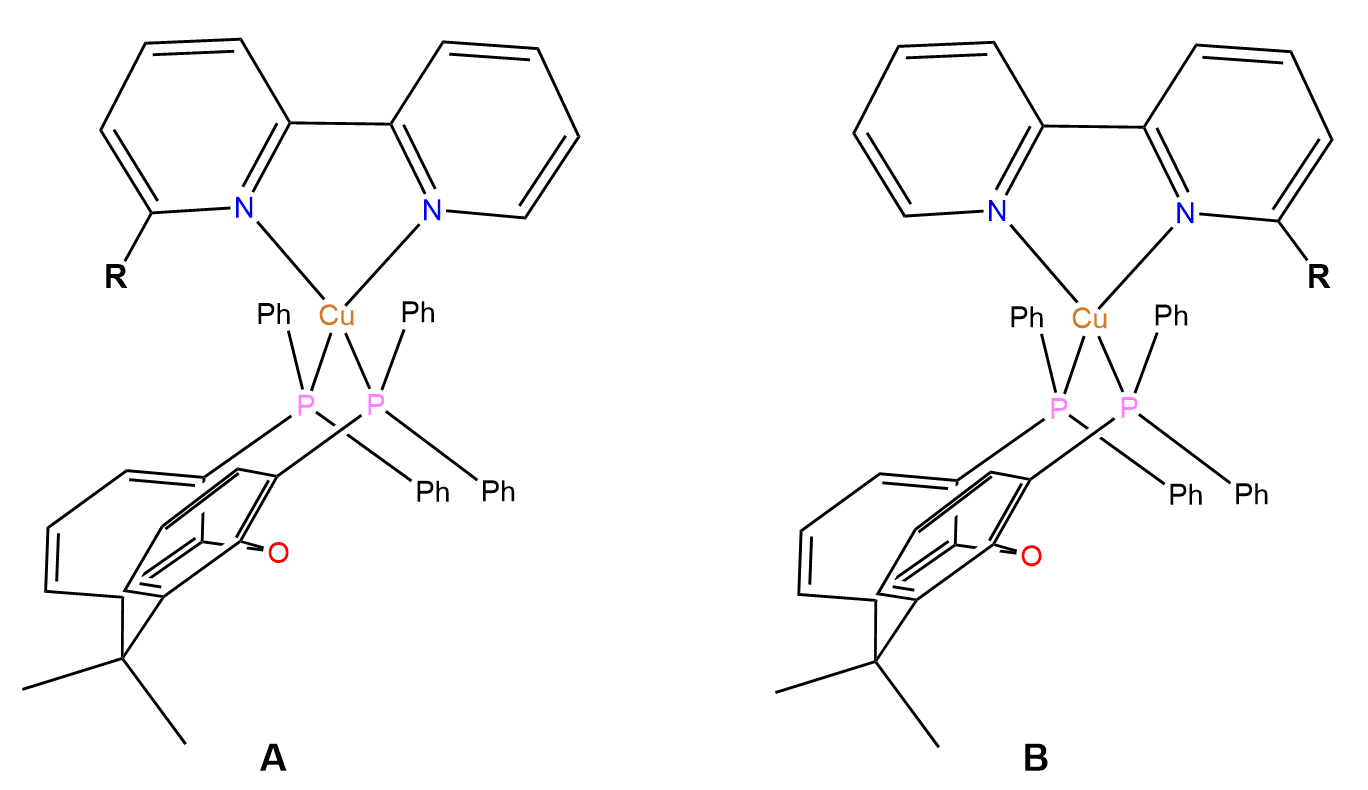
Aiming to introduce stabilizing intramolecular π-stacking interactions in the complexes and enhance their emissions, the scientists decided to investigate the effects of the incorporation of N^N ligands with terminal alkyne moieties (C≡CH) in compounds with formula [Cu(N^N)(P^P)][PF6].
Reported in Figure 2 are the N^N and P^P ligands that were combined in this work to synthesise the six new copper(I) heteroleptic compounds.
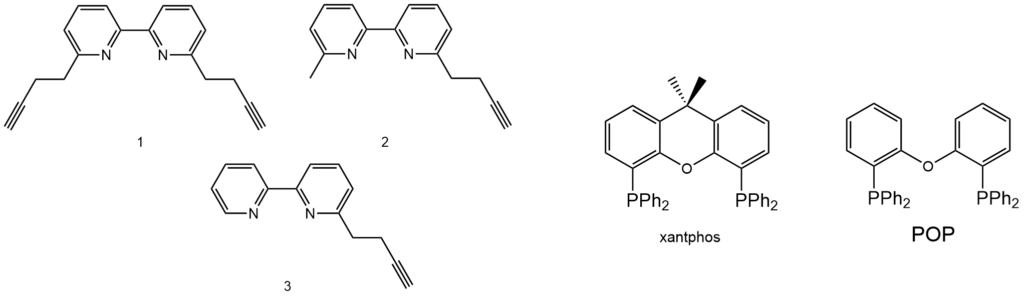
Results
After successfully synthesising the complexes, the team analysed their emission properties and crystal structures. Only for the complexes involving the N^N ligands 1 and 3, and the P^P ligands xantphos and POP, good quality single crystals suitable for X-ray analysis were obtained.
Interestingly, for all the four compounds, near-parallel alignment of one or two C≡CH units over an arene ring were seen. Reported in Figure 3 is an example of this alignment between the CH2CH2C≡CH substituent and the xanthene bowl-shaped cavity of [Cu(3)(xantphos)][PF6].
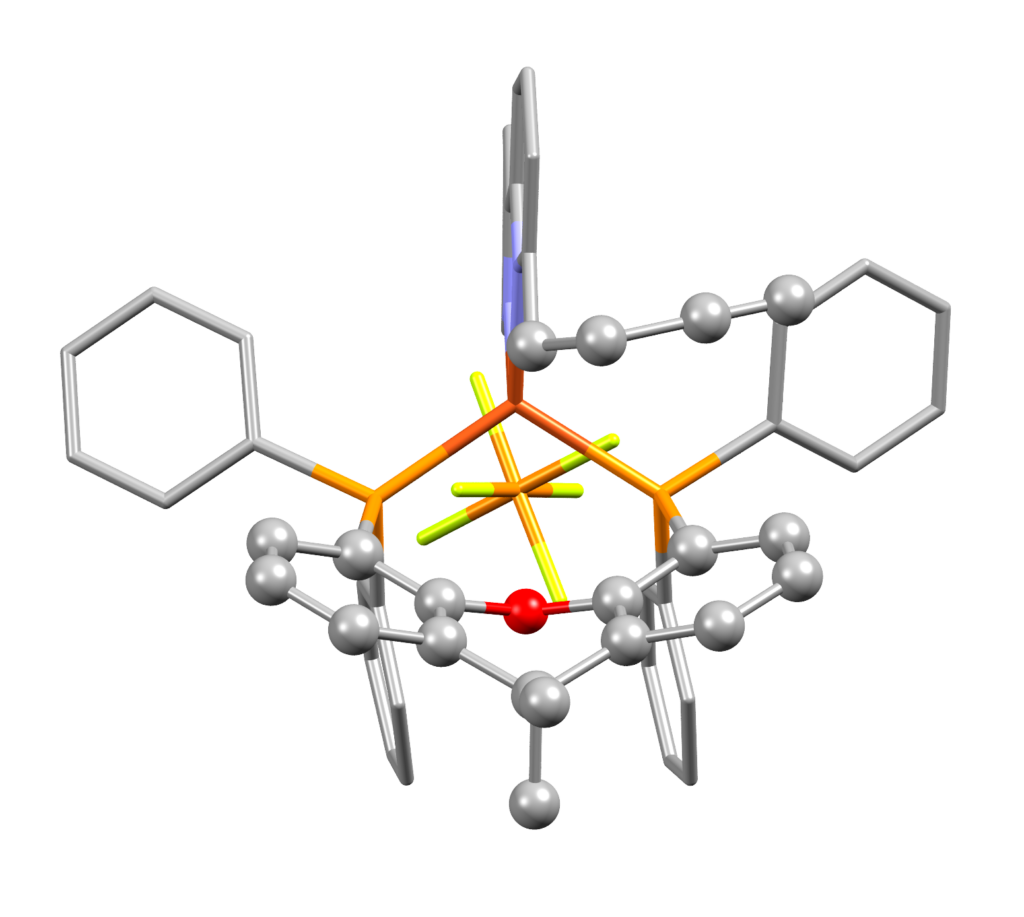
The team decided to search the CSD for intramolecular C≡C⋯arene contacts using ConQuest and found that the C≡C⋯arene distances observed in the new complexes fell well within the expected ranges for related compounds.
It can be concluded that the C≡C⋯arene distances represent meaningful contacts that contribute to the stability of the copper(I) complexes and influence their photophysical properties.
The team hence provided new structural guidelines for guiding the design of new heteroleptic copper(I) compounds exhibiting high PLQYs.
The Tools
ConQuest provides access to the wealth of structural knowledge contained in the CSD and allows users to carry out advanced searches that includes multiple search options (substructure, reference, unit cell, and text terms).
In this work, the team accessed ConQuest to help analysing the stabilizing interactions in existing [Cu(N^N)(P^P)]+ compounds and guiding the design of new copper(I) heteroleptic compounds. The group used this software also to look at the expected ranges for C≡C⋯arene distances, confirming that these interactions are meaningful and influence the stability and emission properties of the newly designed compounds.
Next Steps
Don’t miss the CCDC’s ConQuest playlist on YouTube to discover all about how to conduct searches of the CSD using ConQuest.
To discuss further and/or request a demo with one of our scientists, please contact us via this form or .