FDA Novel Drug Approvals 2022 — Nearly 60% Small Molecules
The Food and Drug Administration (FDA) in the US approved 37 new drugs in 2022, considerably lower than the 50 approvals in 2021, yet still higher than the historic average of 34 approvals per year observed since 1993.
As reported in Nature Reviews Drug Discovery, 21 of the 37 drugs approved were small molecules (including peptides of up to 40 amino acids in length), representing 57% of the total. This figure shows the continued relevance, impact, and value of this class of drugs.
Cancer drugs dominated with 10 new oncology drugs. New drugs to treat diabetes, obesity, psoriasis, and an mRNA-based COVID-19 vaccine were particularly noteworthy for their ‘new target space, new clinical opportunities and untapped commercial potential.’
Availability in the Cambridge Structural Database
Of the 21 small molecule drugs approved, 4 are available in the Cambridge Structural Database (CSD) at the time of writing. All of these structures can be viewed via WebCSD here.
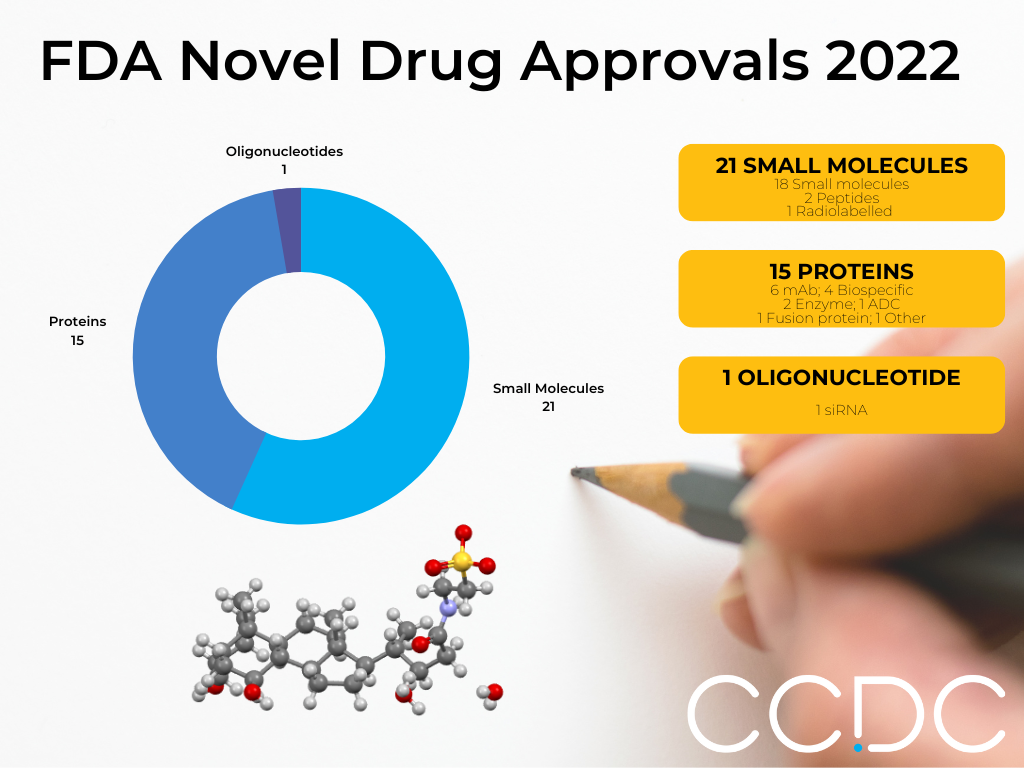
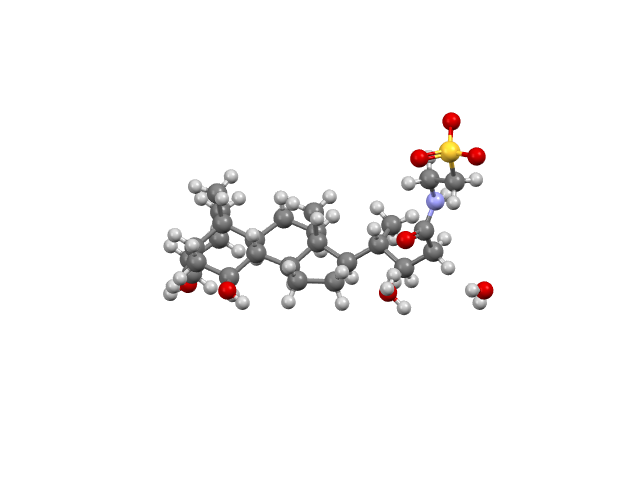
Drug (Brand Name) – Futibatinib (Lytgobi)
Futibatinib is an FGFR kinase inhibitor used to treat FGFR2-aberrant intrahepatic cholangiocarcinoma. CSD refcode: XISCUA.
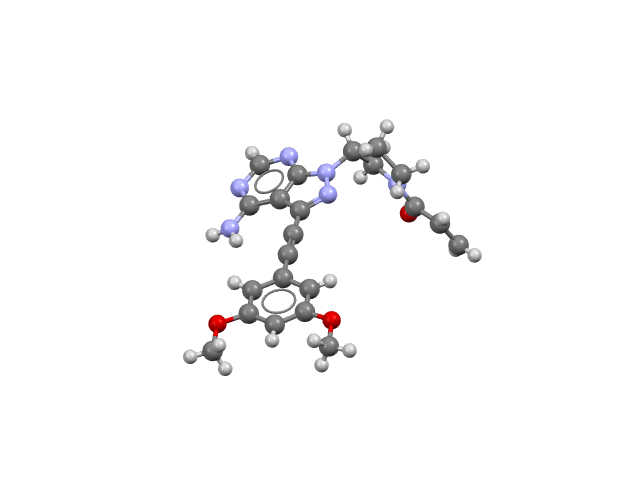
Vonoprazan; Amoxicillin; Clarithromycin (Voquezna Triple Pak)
Voquezna combines a potassium-competitive acid blocker, a penicillin class antibacterial, and a macrolide antimicrobial. It is used to treat Helicobacter pylori infection.
CSD refcodes for the individual co-packaged components: UCIROR (vonoprazan fumarate); AMOXCT10, AMOXCT11, AMOXCT12 (amoxicillin trihydrate); NAVSUY, NAVSUY01, NAVSUY02, and NAVSUY04 (clarithromycin).
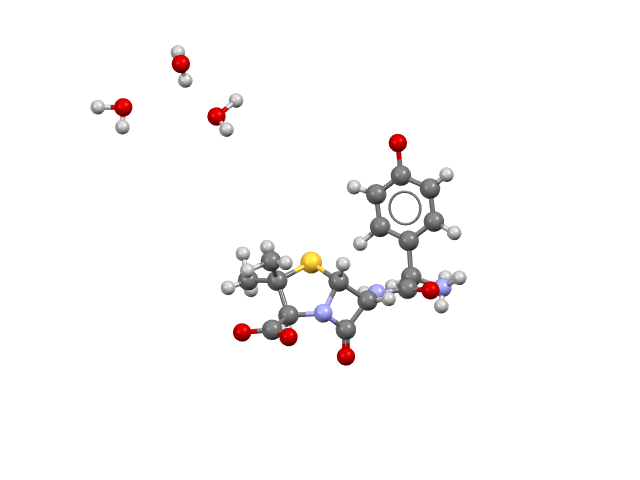
Ganaxolone (Ztalmy)
Ganaxolone is a GABAA receptor positive allosteric modulator used to treat seizures associated with childhood disintegrative disorder (CDD), also known as Heller’s syndrome and disintegrative psychosis. CSD refcode: RIGSOP.
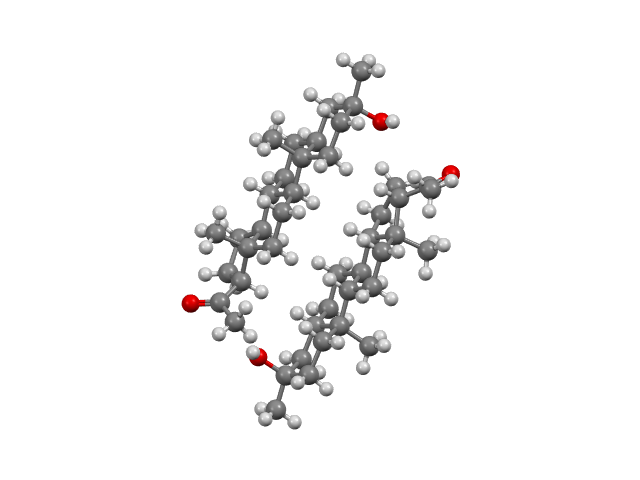
Sodium Phenylbutyrate; Taurursodiol (Relyvrio)
Relyvrio is used to treat amyotrophic lateral sclerosis. CSD refcodes: DIWQIJ and DIWQIJ01 (oxonium salt monohydrate).
Strong Vote for the Reliability of Computational Methods
Owing to animal testing being no longer required by the FDA before human drug trials can begin (legislation signed by President Joe Biden in late December 2022 – read the article in Science here), the reliability of computational methods drawing on quality, validated data sets like the CSD will increasingly become a powerful step in drug discovery. Combined with the seemingly unstoppable rise in AI, will the number of small molecule drug approvals increase over the coming years? Watch this space.
Next Steps
Read the article 2022 FDA approvals in Nature Reviews Drug Discovery.
Learn more about the CSD — the most comprehensive repository of validated and curated small-molecule organic and metal-organic crystal structures.
Learn more about the software provided by the CCDC to get the most out of the data in the CSD.