ConQuest in Action: UBE Industries and Santen Pharmaceutical Co. Finding EP2-selective receptor agonist scaffold for treatment of glaucoma
Here we highlight a recent paper by researchers at UBE Industries and Santen Pharmaceutical Co in which ConQuest helped identify a scaffold with a promising EP2-selective receptor agonist that may lower intraocular pressure (IOP). A prodrug of it was selected as a clinical candidate for the treatment of glaucoma.
This is part of our series highlighting examples of the Cambridge Crystallographic Data Centre (CCDC) tools in action by scientists around the world.
Summary
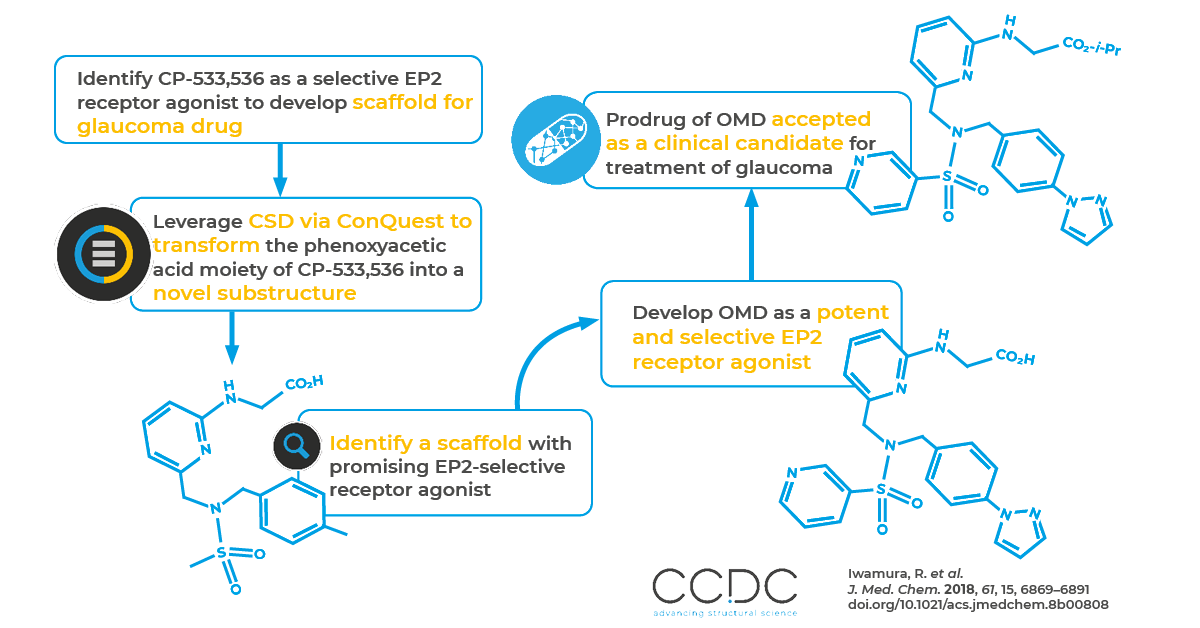
Prostaglandin E2 (PGE2) is a highly bioactive molecule associated with IOP reduction. In this work, researchers explored EP2-receptor agonists to lower IOP. They identified CP-533,536 as a potential scaffold and used ConQuest to identify the prefered torsion angles and conformations for the phenoxyacetic acid moiety of CP-533,536. They then designed new compounds mimicking the preferred conformers. This led to the discovery of (6-((4-(pyrazol-1-yl)benzyl)- (pyridin-3-ylsulfonyl)aminomethyl)pyridin-2-ylamino)acetate, or OMDI. OMDI is now a clinical candidate for the treatment of glaucoma.
How
The researchers followed a two-step process that involved optimizing promising compounds based on statistical analyses of data in the CSD. First, they explored the chemotypes with activities higher than that of the starting compound by transforming the phenoxyacetic acid moiety of CP-533,536 into a novel substructure. Then they transformed pyridin-3-ylsulfonyl and tertbutylphenyl moieties using chemotypes containing highly active novel substructures based on their findings in the first step.
One compound, 13aa showed more potent agonist activity than CP-533,536. Based on the statistical analysis with ConQuest, the researchers hypothesized that the higher h-EP2 receptor agonist activity of 13aa may be due to the physicochemical properties of the pyridyl nitrogen atom (often the characteristic of a hydrogen-bonding acceptor) and the tendency for a 2-aminopyridyl group to have a high level of planarity. Based on that finding, the researchers further optimized the tert-butylphenyl moiety and identified (6-((4-(pyrazol-1-yl)benzyl)(pyridin-3-ylsulfonyl)- aminomethyl)pyridin-2-ylamino)acetic acid – 13ax (OMD) – as a potent and selective h-EP2 receptor agonist. OMDI is a prodrug of OMD.
Why
ConQuest is the primary program for searching and retrieving information from the CSD using an extensive range of flexible search options. Here, the researchers were able to understand and optimize the phenoxyacetic acid moiety of CP-533,536 into a novel scaffold using the tool’s 3D geometric searching to analyse molecular dimensions and determine conformational preferences. This enabled the researchers to optimize substructures based on the over one million structures in the CSD to design a new ocular hypotensive agent with a different mechanism of action than current FP receptor agonists.
Prostaglandin analogues, such as latanoprost, and their active acid forms are selective FP receptor agonists and potent IOP-lowering agents for the treatment of glaucoma. However, therapeutics with new mechanisms of action are needed for cases when patients experience adverse reactions or when concomitant treatment may further reduce IOP. The researchers identified CP-533,536 as a starting point for their scaffold research because it is a selective EP2 receptor agonist under a different clinical trial for fracture healing.
Read More
Read the full paper: Identification of a Selective, Non-Prostanoid EP2 Receptor Agonist for the Treatment of Glaucoma: Omidenepag and its Prodrug Omidenepag Isopropyl (J. Med. Chem. 2018, 61, 6869−6891).
Learn more about ConQuest’s extensive range of flexible search options to access the knowledge contained in over one million crystal structures.
Explore more examples of CCDC tools in action.