Dexamethasone briefing – its chemistry and history
On 16th June news outlets around the world reported the fantastic results seen in trials using dexamethasone in critically ill COVID-19 patients. This quick briefing explores the chemistry and history of this compound, and what could be coming next.
Please note that this article is not intended as medical advice! Like all steroids, dexamethasone carries many side effects and risks, and should only be taken when prescribed by a qualified medical professional.
A quick history of dexamethasone
- August 1958: a new synthetic corticosteroid is reported and tested against Rheumatoid Arthritis by Bunmin et al. The authors note it as; “the most potent of any steroid heretofore studied”
- October 1958: an oral tablet form by MERCK is approved by the FDA.
- 1959: an intravenous form enters the market, also by MERCK
- 1960: a liquid ophthalmic form enters the market, also by MERCK
- 1964: Organon International launches an intravenous form, the first of a series of forms launched by large and small pharmaceutical companies over the next 30 years.
- 1971: The first crystal structure of pure dexamethasone is published by G. van den Bossche and added to the CSD (Refcode DEXMET01)
- 1977: the second crystal structure of pure dexamethasone is published, this time with co-ordinates by GD. C. Rohrer, W. L .Duax and added to the CSD (Refcode: DEXMET10)
- 2002: The structure of dexamethasone bound to the Glucocorticoid receptor is reported by Bledsoe et al., improving our understanding of the drug’s selectivity.
- 2007: The most recent crystal structure of pure dexamethasone is published by J. W. Raynor, W. Minor, M. Chruszcz and added to the CSD (Refcode DEXMET11)
- 2000-2017: with more generic forms available and its applications better understood, around 1 million prescriptions of dexamethasone are made each year in the USA. Its rank moves between the 119th and the 5th most prescribed medication.
- Late 2019: novel coronavirus COVID-19 emerges.
- January 2020: WHO declares the outbreak a public health emergency
- March 2020: WHO declares the outbreak a pandemic
- March 2020: the RECOVERY trial begins in the UK led by the University of Oxford with collaborators and funders including UKRI, NIHR, Wellcome, the Bill and Melinda Gates Foundation and many more. 11,500 patients are enrolled to explore a variety of drugs as treatments for COVID-19 – of which 2104 were randomised to receive dexamethasone.
- June 2020: results are announced that show dexamethasone reduced deaths in ventilated patients and those receiving oxygen.
The chemistry of dexamethasone
- Dexamethasone is a corticosteroid, often referred to simply as steroids.
- Corticosteroids are synthetic drugs which are structurally similar to the biological hormone cortisol, which is produced in the adrenal gland in response to stress.
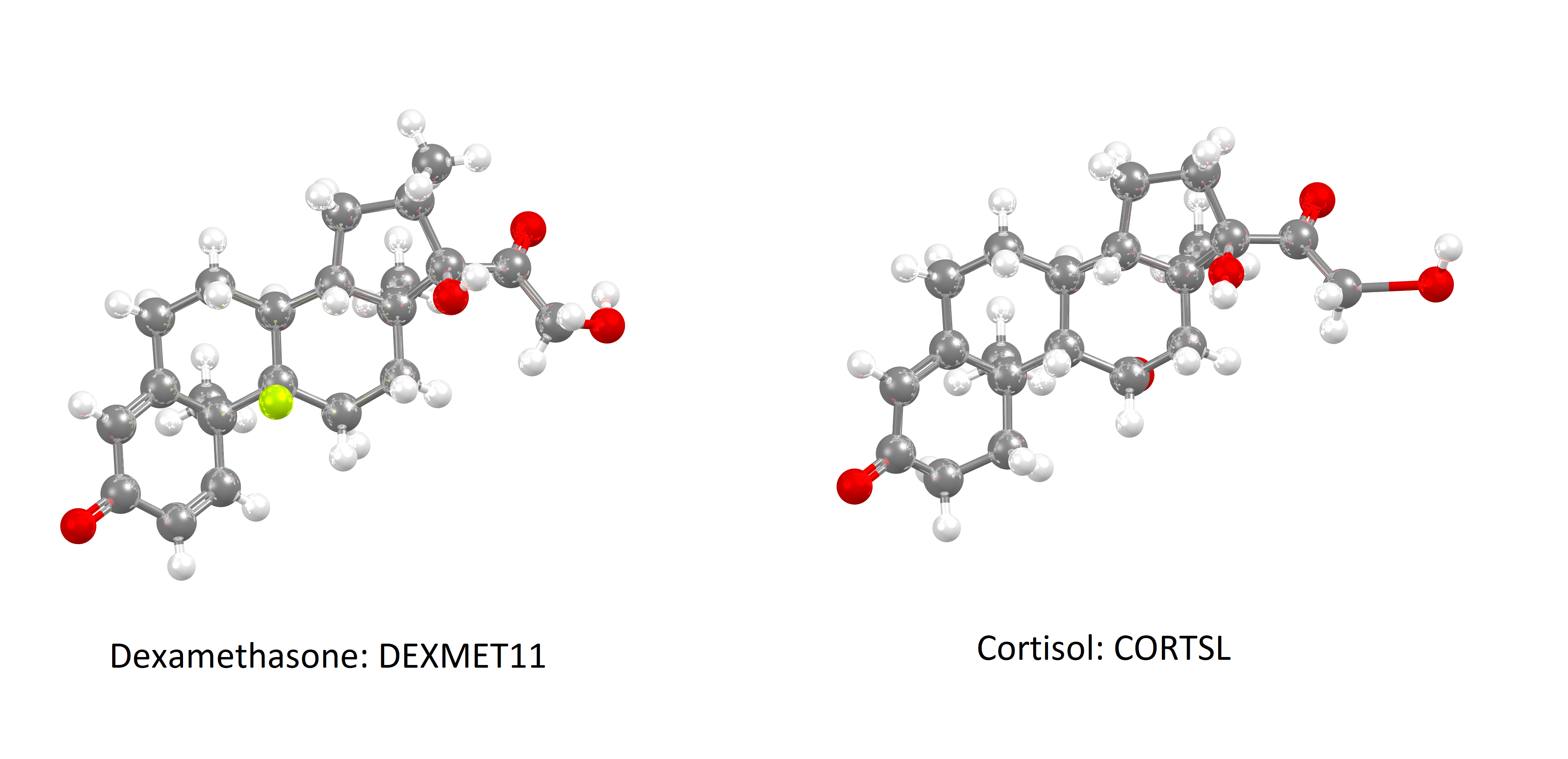 Left: structure of synthetic corticosteroid dexamethasone DEXMET11
Left: structure of synthetic corticosteroid dexamethasone DEXMET11
Right: structure of human stress hormone cortisol CORTSL
- At the time of writing, 24 forms of the drug are listed with the FDA, some of which are discontinued. These include oral tablets, injectable and suspension forms.
- The CSD contains at least 8 crystal forms of the drug, including various multicomponent systems.
- The RECOVERY trial is also exploring other drugs, including Lopinavir-Ritonavir, Azithromycin (GEGJAD02) and Tocilizumab.
What’s next?
While these dexamethasone results are fantastic, this is not the end of the story.
Around the world trials continue, with over 1,400 ongoing at the time of writing. Discovery efforts continue in both pharma companies and crowd-sourced projects. The PostEra COVID Moonshot project for example has had thousands of submissions and is now reporting ever greater progress in their screening and activity analyses.
See our other blogs on COVID-19 to learn about the research CCDC are working on in this area, and how you can get involved.