CSD-Particle in Action: Linking Crystal Structure to Particle Behaviour
Here we highlight a paper by Emilia Prandini and co-workers from Politecnico di Torino and the CCDC.
The team analysed the surface properties of a novel quercetin solvate using the particle informatics tools in CSD-Particle, and validated their findings with experimental techniques.
Particle Informatics and Quercetin
Particle informatics tools help understand the correlation between a crystal structure and particle behaviour, and hence reduce drug development times.
The tools enable users to:
- Analyse intermolecular interactions and predict mechanical properties
- Calculate and inspect the particle morphology
- Evaluate surface energy and surface interactions
The research uses particle informatics tools and experimental techniques to analyse a complex organic structure: a quercetin solvate with N,N-dimethylformamide (DMF), abbreviated QDMF.
Quercetin is a flavonoid found in fruits and vegetables that has antioxidant, anti-inflammatory, and potential antitumor properties. To fully exploit the beneficial effects of quercetin, the scientific community is studying ways to improve its poor bioavailability and low aqueous solubility.
Quercetin is hence a good candidate molecule to investigate how structural features affect particle properties.
How the Analysis Was Performed
The team started by analysing the crystal structure of the QDMF solvate collected at 173 K, which crystallizes with one quercetin molecule and two DMF molecules in the asymmetric unit.
The crystal packing showed hydrogen bonds between quercetin–quercetin and quercetin–DMF molecules, Figure 1 (a), with molecules arranged in undulated ribbons, Figure 1.
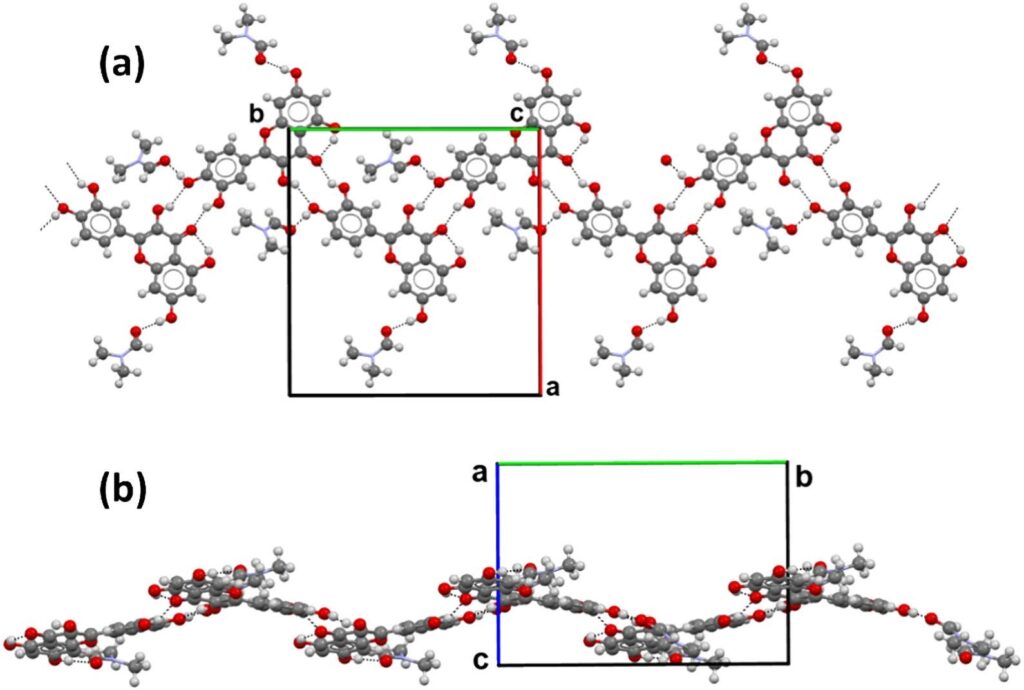
The Void calculation performed with Mercury led to the identification of the volume occupied by the molecules of DMF, which corresponds to 23.9% of the unit cell volume.
By combining differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), two desolvation processes were identified at 103 °C and 171 °C. These high temperatures suggested that the DMF molecules were strongly bound in the crystal structure.
The team also followed the structural changes of QDMF upon heating with variable temperature powder X-ray diffraction (VT-PXRD), confirming that the original structure is not preserved when the solvent is removed, and that an anhydrous form of quercetin is instead obtained.
The Intermolecular Interactions Analysis
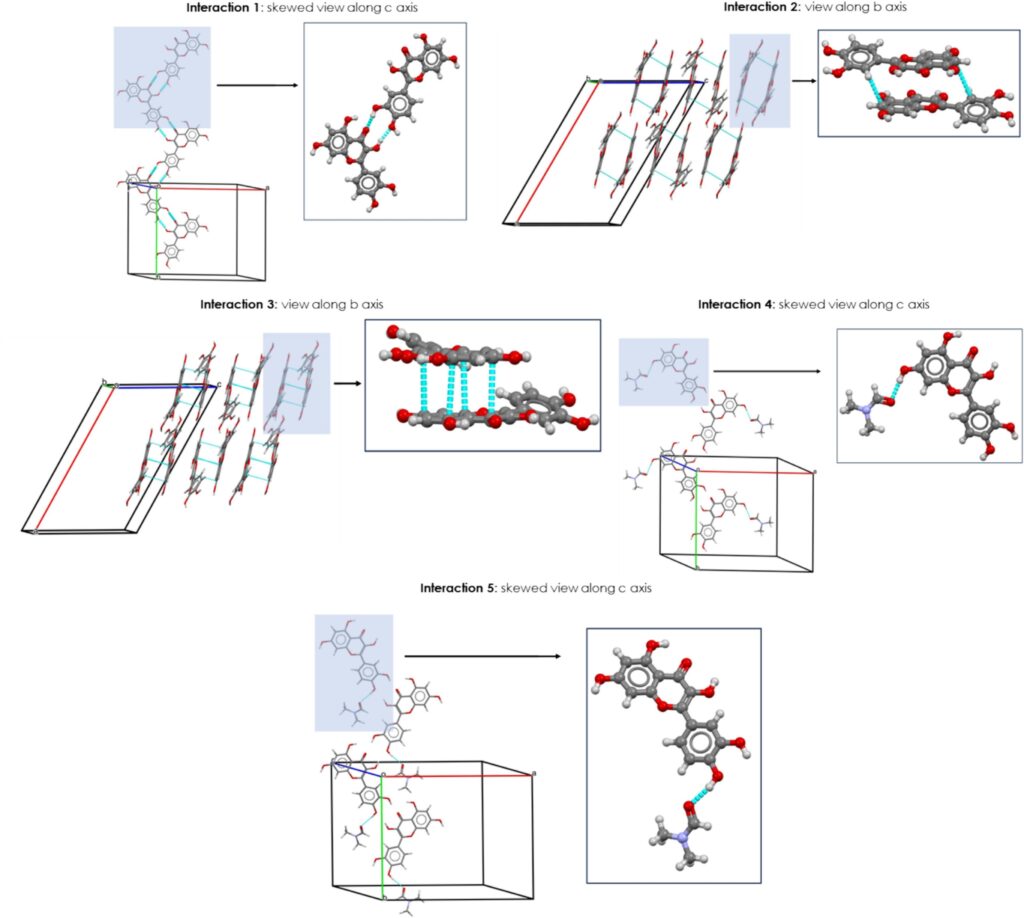
The Visual Habit module included in the CSD-Particle suite of Mercury was used to analyse the interaction energies of the synthons in QDMF.
The analysis led to the identification of the five strongest intermolecular interactions (Figure 2), contributing to 75.9 % of the total lattice energy. These interactions are here listed, with the percentage of the contribution to the total lattice energy indicated in brackets:
- Hydrogen bond between two quercetin molecules (interaction 1, 19.4%)
- Two different off-set stacking interactions between two quercetin molecules (interactions 2 and 3, 18.9% and 16.9%)
- Two different hydrogen bonds between one molecule of quercetin and the two molecules of DMF (interactions 4 and 5, 10.5% and 10.2%)
The latter involve two different hydroxyl groups of the quercetin molecule and two different molecules of DMF. These interactions are similar in energy but differ slightly in the geometry (centroids position), explaining the two-steps desolvation process seen by DSC and TGA: the DMF molecule in interaction 5 (Figure 2) is released first, followed by the one involved in interaction 4 (Figure 2).
The Crystal Surface Analysis
Using the attachment energy model in Mercury on the experimentally indexed crystal, it was seen that the stacking interactions between quercetin molecules contribute to the growth of the (001) facet (Figure 3).
Hydrogen bonding interactions between quercetin molecules contribute instead to the growth of the other two main exposed facets, the (1-10) and (200) facets (Figure 3). This was further confirmed using the Surface Analysis tool in Mercury, which showed fewer aromatic groups in these facets. These calculations matched with the experimental values of contact angle measured, which indicated a hydrophilic nature for the facets.
The rugosity of the surfaces was also calculated, showing that the facet with higher rugosity is the (200), but that none of the facets can be considered smooth (Figure 3).
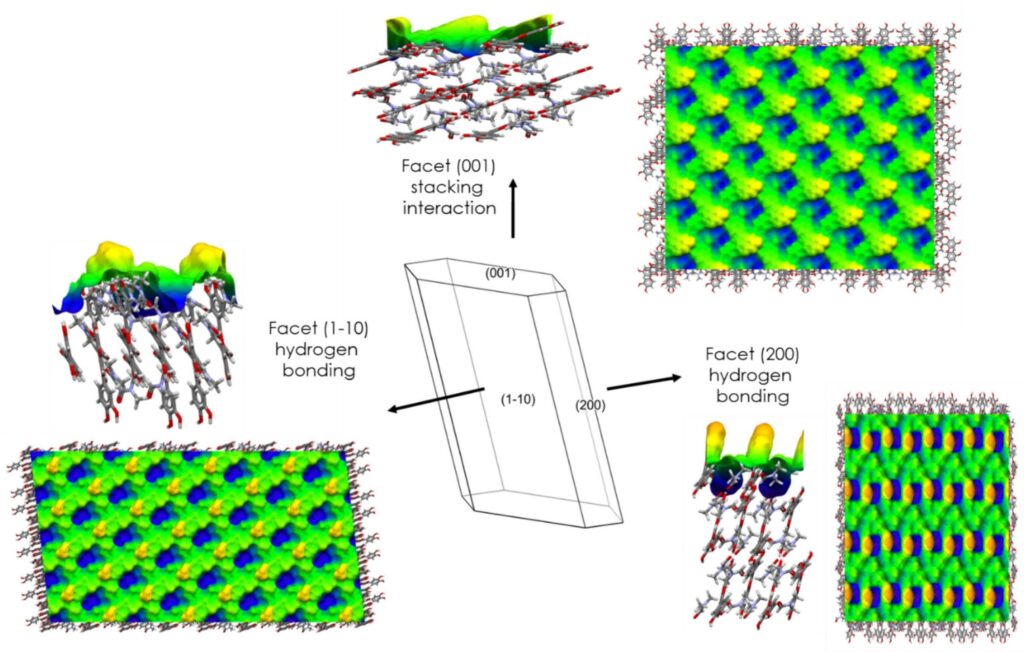
The largest (1-10) facet is characterized by a high percentage of DMF molecules, explaining why the desolvation occurs preferentially on this facet. This was confirmed with Scanning Electron Microscopy (SEM) and Raman spectroscopy experiments.
Conclusion
The particle informatics tools included in CSD-Particle were used with experimental techniques to understand the desolvation behaviour of QDMF.
The results demonstrate that these tools are effective in predicting critical particle properties.
Next Steps
To discuss further and/or request a demo with one of our scientists, please contact us via this form or .