CSD-CrossMiner in action: exploring molecular scaffolds for a synthesized MCR library
Here we summarise a recent paper by Dr Markella Konstantinidou and co-workers in which CSD-CrossMiner was used to explore further alternate scaffolds of a synthesized library of drug-like compounds based on a little explored multicomponent reaction (MCR).
Summary
The authors were interested in the heterocyclic ring system produced by the three component Groebke-Blackburn-Bienaymé reaction as a possible pharmaceutical scaffold. They designed and synthesized a library of substituted products, and solved the structure of one to confirm its structural properties. CSD-CrossMiner was used to identify binding patterns (as it is seen both in their obtained crystal structure and the literature), to find similar structural motifs in the PDB and generate new ideas for further lead optimization and scaffold hopping.
Read the article here: Konstantinidou, M. et al, 2020, Eur. J. Org. Chem., 2020: 5601-5605.
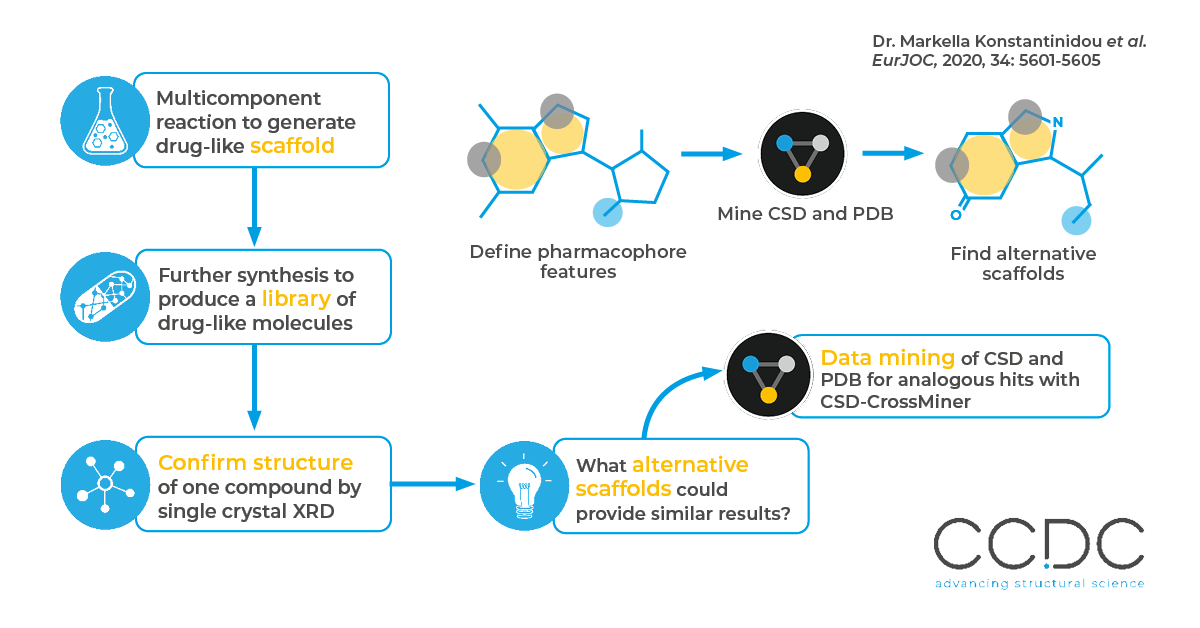
Why
The ability to scaffold hop, that is to change a structure whilst retaining the key features which make it biologically active, allows medicinal chemists to design possible alternatives. This may be to improve the pharmacology or specificity.
How
The authors used CSD-CrossMiner to define a pharmacophore search against a reference PDB structure with very similar binding pattern to their scaffold. This was done by defining the key features essential to the molecule’s function, and then mining the PDB for results which fulfil these features. This returned 37 cocrystal structures, demonstrating additional potential future scaffolds.
Read more
Read the European Journal of Organic Chemistry paper here.
Learn more about CSD-CrossMiner here.
Explore more examples of CCDC tools in action here.
Download the Infographic CrossMiner: Drug Discovery through Data Mining