Accelerating the Molecule to Medicine Journey Through the Application of Structural Science
This blog is based on the webinar “From Molecule to Medicine – a Journey” presented by Dr Robert Docherty. In the webinar, Dr Docherty demonstrated the impact of crystallographic data on drug development, from discovery to manufacturing, and their potential to accelerate digital transformation in the pharmaceutical industry.
Dr Robert Docherty started his career as a computational chemist, and later joined Pfizer as head of Materials Sciences in Drug Product Design. He then started developing his research and worked on strategic lead for digital design at Pfizer. Currently, Dr Docherty is a CCDC consultant for the Materials Science Team, with a particular focus in particle and solid form informatics.

Background and Context – Why
Pharmaceutical materials science is pivotal to accelerate the process of development of a new drug. The solid form of a particle strongly influences its properties (e.g. dissolution, stability, and homogeneity), affecting the safety, efficacy, and manufacturing efficiency of the product. Hence, getting the “right” solid form and particle attributes, particularly at an early stage, is fundamental to accelerate the molecule to medicine journey.
Many challenges associated with unexpected solid form issues have been encountered in the pharmaceutical industry [1]. In 1998, the HIV protease inhibitor Norvir (ritonavir) was withdrawn from the market due to dissolution failure of the oral capsules caused by the appearance of a more thermodynamically stable form. In 2008, Schwarz Pharma recalled the Neupro (rotigotine transdermal) patches due to the crystallization of a new polymorph in the patches.
A lot has been learnt from these challenges. Nowadays there are numerous case studies where the preferred form that reached development and manufacturing is the most stable one, requiring solubility enhancement studies to be done. In other cases, the chosen form is a metastable one, and studies to manage the long-term stability control had to be put in place.
Structural data are integral to the product design and are becoming even more important as we move towards a digital framework for product and process development which will revolutionize product realization in terms of speed and quality.
Phases of Development – When
The drug discovery and development timeline for a solid form is divided into three phases, and each of these require different tools. The discovery phase requires fast, qualitative, and intuitive tools to use in partnership with medicinal chemists to select the right molecule and properties. State-of-the-art techniques, tools, and expertise are used in the second phase to design the right solid form particle and product. Solid form characterization is the final step and requires consistent and well understood methods to underpin the manufacturing control strategy.
While early phases focus on developing synthetic routes for the API and facilitating rapid clinical progress, the mindset in late stages of development changes to robustness and focus on making sure that the API route is well established and optimized. Within the overall process, the duration, success percentage, and costs for each of the phases need to be taken into consideration, and particle science is crucial to help moving to the next phase and getting the confidence needed to reach the manufacturing phase.
Properties that may be assessed during the selection of a solid form for a standard oral dosage form are its safety, efficacy, and quality. It is important for a solid form to be stable under the conditions of isolation, purification, and storage. The dose administered should dissolve completely, and studies of aqueous and pH solubilities should be present. It should be easy to ensure consistent product attributes, with no changes of hydrate state under storage conditions and physical stability to milling and compaction.
Fusing Experimental and Digital Workflows – How
Structural science can help selecting the right molecule, the right design, and optimizing the manufacturing processes. How?
An interesting example of a study around low solubility was reported in the Journal of Pharmacy and Pharmacology [2]. The team presented a model which de-convolutes the solubility of pharmaceutical compounds into solvation and packing properties, aiming to identify the cause of low solubility in 54 pharmaceutical compounds. The Cambridge Structural Database (CSD) was the source of structural information.
Another example is a study that was reported in the Journal of Pharmaceutical Sciences, in which crystallographic information were used to build a machine learning algorithm to estimate the lattice energies [3].
The stability of a solid form is critical when thinking about the transition into a drug product. A work published in Crystal Growth and Design reports the study of the hydration kinetics of three anhydrous polymorphs of fluconazole [4]. On top of the classical material science analyses (PXRD, DSC, SEM), the structural analysis allowed the scientists to follow the changes upon hydration, and to investigate the impact of crystal structure and molecular conformation on the kinetics of hydration. This represents a nice example of what a physical stability workflow could look like.
Finally, at the product stage, researchers start looking at the critical pieces of information that come from analysing the particle and the interaction of solvents or other ingredients with the particle.
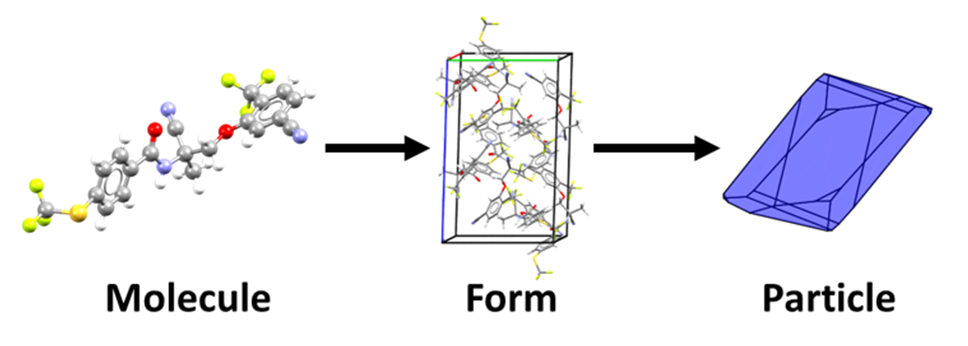
In this work reported in Crystal Growth and Design, scientists looked at the particle properties of the antiepileptic drug lamotrigine [5]. Using a combination of computational and topological methods, they analysed its intermolecular interactions, mechanical properties, morphology, and surface energy. The team then used this information to rationalize several issues encountered during the formulation and manufacturing of lamotrigine.
Conclusions
Crystallographic information is critical in the development process, not only to confirm the molecular structure and to connect the structure to the properties of the API, but also to optimize the synthetic process. They are important for getting an understanding of the solid form landscape and avoiding solvate and hydrate formation, and are vital to perform solid form control tests.
A clear understanding of solid form and particle attributes is crucial to accelerated product development strategies.
Next Steps
To discuss further and/or request a demo of how the Cambridge Structural Database (CSD) can accelerate and guide your research with one of our scientists, please contact us via this form or .
References
[1] Annu. Rev. Chem. Biomol. Eng. 2, 259–280 (2011).
[2] Journal of Pharmacy and Pharmacology 67(6), 847-856 (2015).
[3] Journal of Pharmaceutical Sciences, 108(10), 3176-3186 (2019).