Xenon
Xenon:
Xenon is the most popular gas used in flashlamps because of its good efficiency. It converts nearly 50% of electrical energy into light.
Facts about Xenon:
- Xenon: Colourless, odourless and dense gas at standard conditions
- Fun fact about Xenon: Xenon has applications in aircraft propellant, medical imaging, lasers and nuclear energy.
- Chemical symbol: Xe
- Atomic number: 54
A crystal structure containing Xenon:
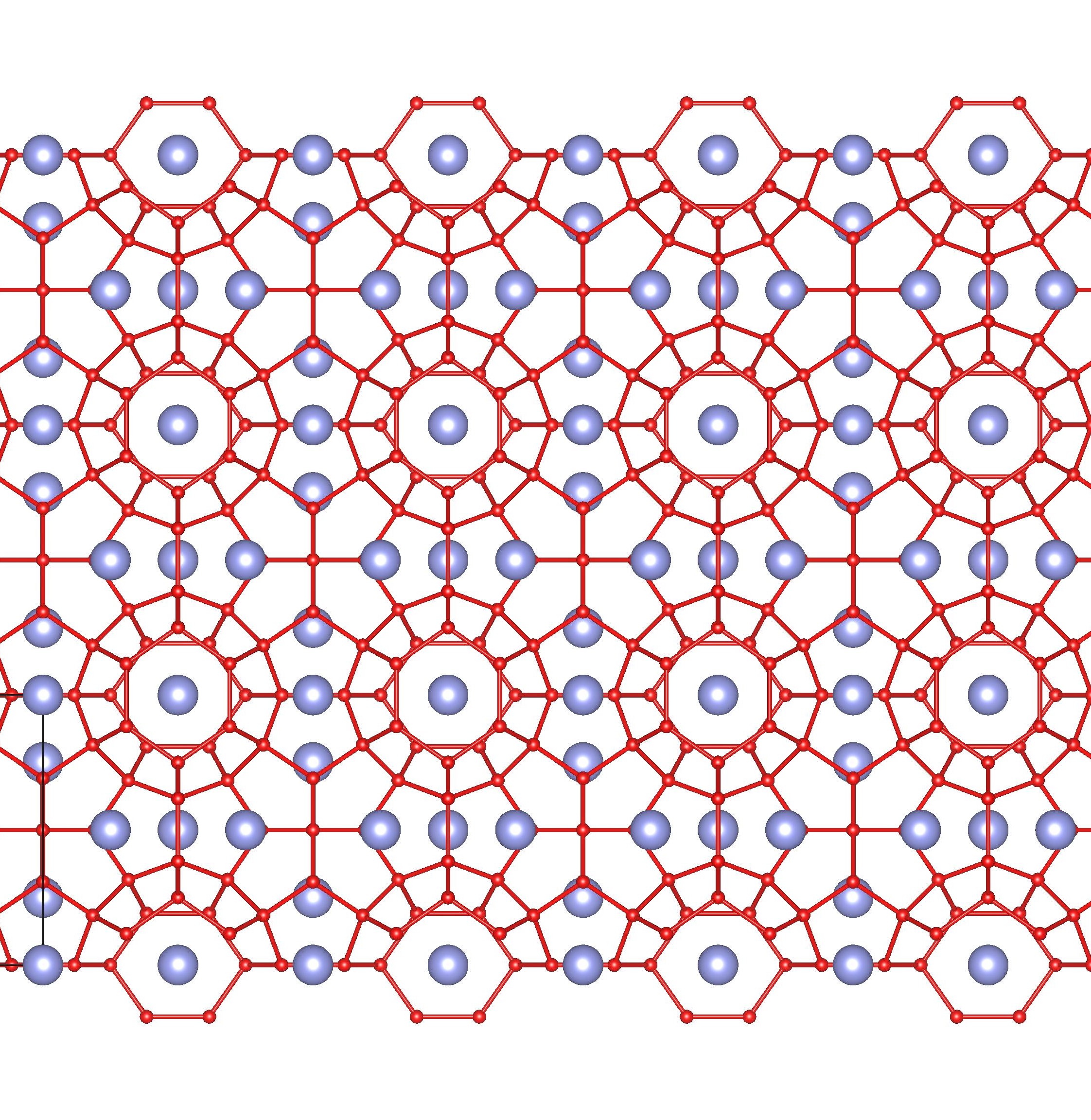
Connected red spheres represent oxygen atoms in the many water molecules that fuse hydrogen bonded cages, light blue spheres the xenon atoms trapped in the cages.
Facts about this structure:
- Formula: Xe3.22(H2O)23
- Structure name: cubic xenon clathrate hydrate
- Fun fact about the structure: Clathrate hydrates are shown trapping xenon here, but they are found in nature trapping 6.4 trillion (6.4×1012) tonnes of methane on the deep ocean floor!
- ICSD Number: 420421 (Find out more about the ICSD database)
- Associated publication: L. Yang, C.A. Tulk, D.D Klug, I.L. Moudrakovski, C.I. Ratcliffe, J.A. Ripmeester, B.C. Chakoumakos, L. Ehm, C.D. Martin and J.B. Parise. Proceedings of the National Academy of Sciences of the United States of America (2009) 106, (15) p6060-p6064, DOI: 10.1073/pnas.0809342106
More about Xenon:
Xenon, one of the ‘noble gases’ was discovered in 1893 by William Ramsay and Morris Travers who had also discovered its companions neon and krypton. Like all nobel gases, xenon finds it hard to bond with other elements and this can lead to some very interesting structures. When xenon is mixed with water and forced to crystallise (usually under high pressure and low temperatures) then the water, unable to bond with the xenon itself under these conditions, forms cages around it. Known as a ‘clathrate’ hydrate – we’re featuring the sI cubic form. This structure will change under different conditions, and the paper featured describes how this results in new water-cage structures.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Xenon in a real crystal structure: