CSD in Action: Bristol-Myers Squibb. Novel Factor VIIa Inhibitors via Fragment-based Screening
Here we highlight one paper which used fragment based screening to identify new inhibitors with improved pharmacokinetic properties. Part of our series highlighting examples of CCDC tools in action by scientists around the world.
Summary
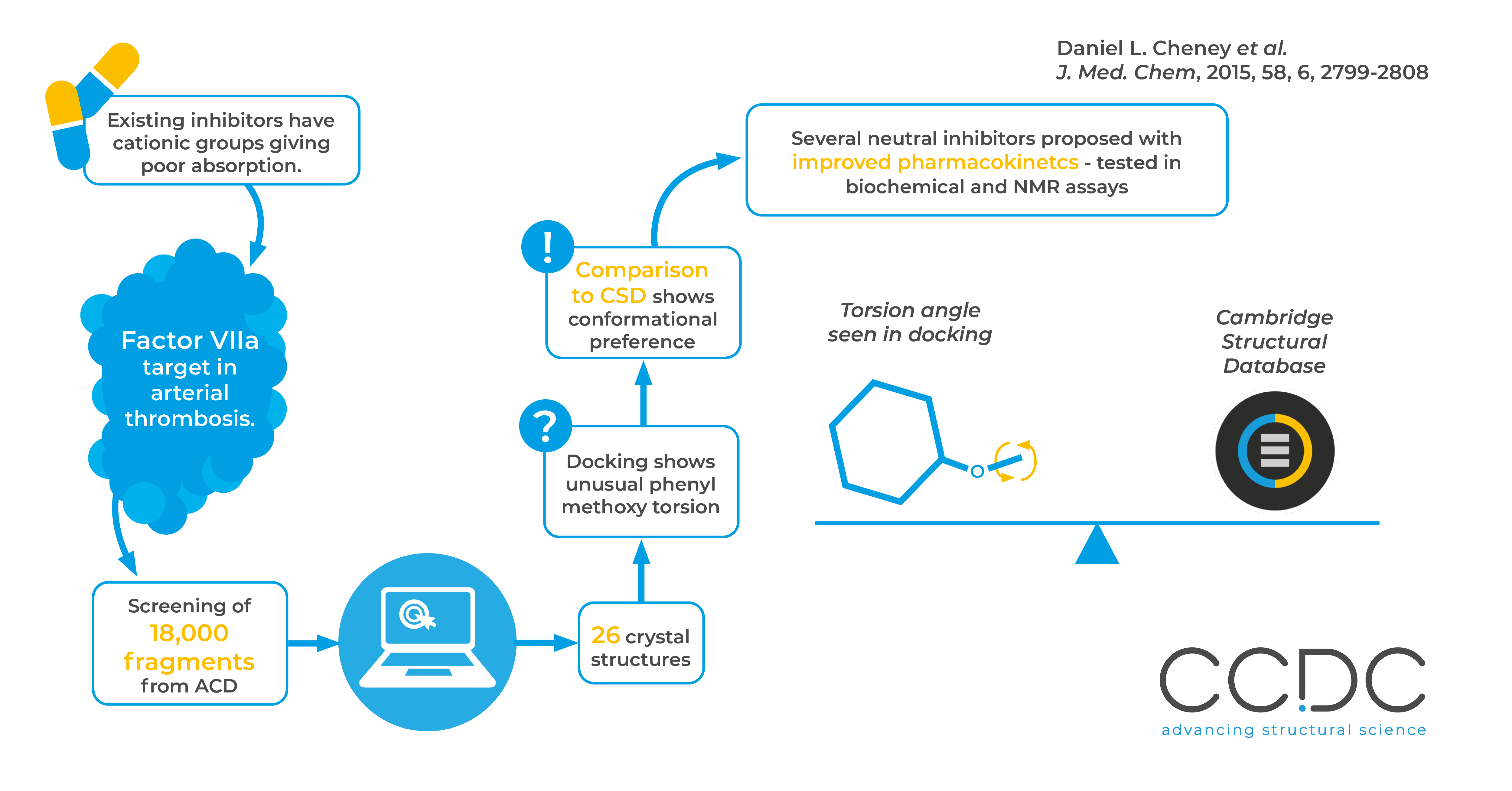
In this work a group at Bristol-Myers Squibb used fragment based screening to find new inhibitors of coagulation factor VIIa. The CSD was used to evaluate conformational preferences, by comparing the C-C-O-C dihedral angle observed in all anisole substructures docking against all experimentally observed instances of this substructure.
Why
Factor VIIa is a serine protease, involved in the coagulation cascade and identified as a target to prevent arterial thrombosis. Known inhibitors contain cationic P1 groups, which give poor pharmacokinetic properties.
If the cationic P1 groups could be replaced by neutral groups, this would improve pharmacokinetic properties of membrane permeability and oral absorption.
How
The group used a multidisciplinary approach combining various computational and lab techniques. The ACD (Available Chemicals Directory) was filtered by drug-like properties, and suitable fragments identified visually and by docking. Fragment-based screening with NMR and biochemical assays then further refined the results.
During docking an unusual torsion was identified, and comparison against the CSD confirmed that only 1.3% of experimentally observed structures had this degree of torsion. This validation of the docking results against real data allowed this unfavourable conformation to be identified early in the process.
Read more
Read the J. Med. Chem. paper here.
Learn more about the CSD here.
Explore more examples of CCDC tools in action here.